CRM1/Ran-mediated nuclear export of p27(Kip1) involves a nuclear export signal and links p27 export and proteolysis
- PMID: 12529437
- PMCID: PMC140238
- DOI: 10.1091/mbc.e02-06-0319
CRM1/Ran-mediated nuclear export of p27(Kip1) involves a nuclear export signal and links p27 export and proteolysis
Abstract
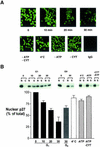
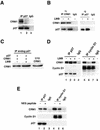
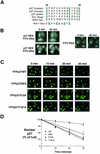
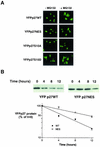
We show that p27 localization is cell cycle regulated and we suggest that active CRM1/RanGTP-mediated nuclear export of p27 may be linked to cytoplasmic p27 proteolysis in early G1. p27 is nuclear in G0 and early G1 and appears transiently in the cytoplasm at the G1/S transition. Association of p27 with the exportin CRM1 was minimal in G0 and increased markedly during G1-to-S phase progression. Proteasome inhibition in mid-G1 did not impair nuclear import of p27, but led to accumulation of p27 in the cytoplasm, suggesting that export precedes degradation for at least part of the cellular p27 pool. p27-CRM1 binding and nuclear export were inhibited by S10A mutation but not by T187A mutation. A putative nuclear export sequence in p27 is identified whose mutation reduced p27-CRM1 interaction, nuclear export, and p27 degradation. Leptomycin B (LMB) did not inhibit p27-CRM1 binding, nor did it prevent p27 export in vitro or in heterokaryon assays. Prebinding of CRM1 to the HIV-1 Rev nuclear export sequence did not inhibit p27-CRM1 interaction, suggesting that p27 binds CRM1 at a non-LMB-sensitive motif. LMB increased total cellular p27 and may do so indirectly, through effects on other p27 regulatory proteins. These data suggest a model in which p27 undergoes active, CRM1-dependent nuclear export and cytoplasmic degradation in early G1. This would permit the incremental activation of cyclin E-Cdk2 leading to cyclin E-Cdk2-mediated T187 phosphorylation and p27 proteolysis in late G1 and S phase.
Figures




References
-
- Adam SA, Sterne-Marr R, Gerace L. Nuclear protein import using digitonin-permeabilized cells. Methods Enzymol. 1992;219:97–110. - PubMed
-
- Askjaer P, Jensen TH, Nilsson J, Englmeier L, Kjems J. The specificity of the CRM1-Rev nuclear export signal interaction is mediated by RanGTP. JBiolChem. 1998;273:33414–33422. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

