Pit membrane porosity and water stress-induced cavitation in four co-existing dry rainforest tree species
- PMID: 12529513
- PMCID: PMC166785
- DOI: 10.1104/pp.014100
Pit membrane porosity and water stress-induced cavitation in four co-existing dry rainforest tree species
Abstract
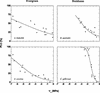
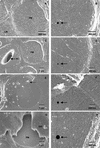
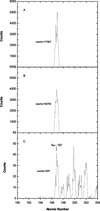
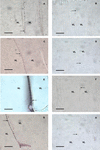
Aspects of xylem anatomy and vulnerability to water stress-induced embolism were examined in stems of two drought-deciduous species, Brachychiton australis (Schott and Endl.) A. Terracc. and Cochlospermum gillivraei Benth., and two evergreen species, Alphitonia excelsa (Fenzal) Benth. and Austromyrtus bidwillii (Benth.) Burret., growing in a seasonally dry rainforest. The deciduous species were more vulnerable to water stress-induced xylem embolism. B. australis and C. gillivraei reached a 50% loss of hydraulic conductivity at -3.17 MPa and -1.44 MPa, respectively; a 50% loss of hydraulic conductivity occurred at -5.56 MPa in A. excelsa and -5.12 MPa in A. bidwillii. To determine whether pit membrane porosity was responsible for greater vulnerability to embolism (air seeding hypothesis), pit membrane structure was examined. Expected pore sizes were calculated from vulnerability curves; however, the predicted inter-specific variation in pore sizes was not detected using scanning electron microscopy (pores were not visible to a resolution of 20 nm). Suspensions of colloidal gold particles were then perfused through branch sections. These experiments indicated that pit membrane pores were between 5 and 20 nm in diameter in all four species. The results may be explained by three possibilities: (a) the pores of the expected size range were not present, (b) larger pores, within the size range to cause air seeding, were present but were rare enough to avoid detection, or (c) pore sizes in the expected range only develop while the membrane is under mechanical stress (during air seeding) due to stretching/flexing.
Figures
References
-
- Alder NN, Sperry JS, Pockman WT. Root and stem xylem embolism, stomatal conductance, and leaf turgor in Acer grandidentatum populations along a soil moisture gradient. Oecologia. 1996;105:293–301. - PubMed
-
- Butterfield BG, Meylan BA. Cell wall hydrolysis in the tracheary elements of the secondary xylem. In: Baas P, editor. New Perspectives in Wood Anatomy. The Hague, The Netherlands: Junk Publishers; 1982.
-
- Crombie DS, Hipkins MF, Milburn JA. Gas penetration of pit membranes in the xylem of Rhododendron as the cause of acoustically detected sap cavitation. Aust J Plant Physiol. 1985;12:445–453.
-
- Cronshaw J. The fine structure of the pits of Eucalyptus regnans (F. Muell.) and their relation to the movement of liquids into wood. Aust J Bot. 1960;8:51–57.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

