Elicitor activity of a fungal endopolygalacturonase in tobacco requires a functional catalytic site and cell wall localization
- PMID: 12529518
- PMCID: PMC166790
- DOI: 10.1104/pp.011585
Elicitor activity of a fungal endopolygalacturonase in tobacco requires a functional catalytic site and cell wall localization
Abstract
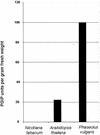
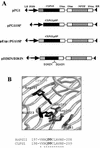
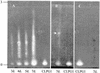
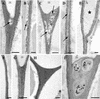
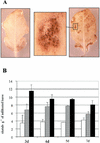
CLPG1, an endopolygalacturonase (endoPG) gene of Colletotrichum lindemuthianum, was transferred to tobacco (Nicotiana tabacum) leaves by using the Agrobacterium tumefaciens transient delivery system. The following four constructs were prepared: CLPG1, with or without its signal peptide (SP; PG1, PG1deltaSP); CLPG1 with the tobacco expansin1 SP instead of its own SP (Exp::PG1deltaSP); and a mutated version of the latter on two amino acids potentially involved in the catalytic site of CLPG1 (D202N/D203N). Chlorotic and necrotic lesions appeared 5 to 7 d postinfiltration, exclusively in response to CLPG1 fused to the expansin SP. The lesions were correlated to the production of an active enzyme. Necrosis-inducing activity, as well as endoPG activity, were completely abolished by site-directed mutagenesis. Ultrastructural immunocytolocalization experiments indicated that the expansin SP addressed CLPG1 to the cell wall. Staining of parenchyma cells revealed the progressive degradation of pectic material in junction zones and middle lamella as a function of time after infiltration, ultimately leading to cell separation. A 30% decrease in the GalUA content of the cell walls was simultaneously recorded, thereby confirming the hydrolytic effect of CLPG1 on pectic polysaccharides, in planta. The elicitor activity of CLPG1 was further illustrated by the induction of defense responses comprising active oxygen species and beta-1,3-glucanase activity, before leaf necrosis. Altogether, the data demonstrate that an appropriate SP and a functional catalytic site are required for the proper expression and elicitor activity of the fungal endoPG CLPG1 in tobacco.
Figures



References
-
- Armand S, Wagemaker MJ, Sanchez-Torres P, Kester HC, van Santen Y, Dijkstra BW, Visser J, Benen JA. The active site topology of Aspergillus niger endopolygalacturonase II as studied by site-directed mutagenesis. J Biol Chem. 2000;275:691–696. - PubMed
-
- Bendahmane A, Querci M, Kanyuka K, Baulcombe DC. Agrobacterium transient expression system as a tool for the isolation of disease resistance genes: application to the Rx2 locus in potato. Plant J. 2000;21:73–81. - PubMed
-
- Blumenkrantz N, Asboe-Hansen G. A new method for quantitative determination of uronic acids. Anal Biochem. 1973;54:484–489. - PubMed
-
- Boudart G, Lafitte C, Barthe JP, Frasez D, Esquerré-Tugayé MT. Differential elicitation of defense responses by pectic fragments in bean seedlings. Planta. 1998;206:86–94.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

