Functional mitochondrial complex I is required by tobacco leaves for optimal photosynthetic performance in photorespiratory conditions and during transients
- PMID: 12529534
- PMCID: PMC166806
- DOI: 10.1104/pp.011155
Functional mitochondrial complex I is required by tobacco leaves for optimal photosynthetic performance in photorespiratory conditions and during transients
Abstract
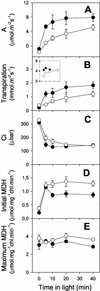
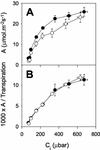
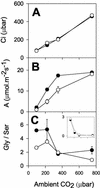
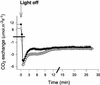
The importance of the mitochondrial electron transport chain in photosynthesis was studied using the tobacco (Nicotiana sylvestris) mutant CMSII, which lacks functional complex I. Rubisco activities and oxygen evolution at saturating CO(2) showed that photosynthetic capacity in the mutant was at least as high as in wild-type (WT) leaves. Despite this, steady-state photosynthesis in the mutant was reduced by 20% to 30% at atmospheric CO(2) levels. The inhibition of photosynthesis was alleviated by high CO(2) or low O(2). The mutant showed a prolonged induction of photosynthesis, which was exacerbated in conditions favoring photorespiration and which was accompanied by increased extractable NADP-malate dehydrogenase activity. Feeding experiments with leaf discs demonstrated that CMSII had a lower capacity than the WT for glycine (Gly) oxidation in the dark. Analysis of the postillumination burst in CO(2) evolution showed that this was not because of insufficient Gly decarboxylase capacity. Despite the lower rate of Gly metabolism in CMSII leaves in the dark, the Gly to Ser ratio in the light displayed a similar dependence on photosynthesis to the WT. It is concluded that: (a) Mitochondrial complex I is required for optimal photosynthetic performance, despite the operation of alternative dehydrogenases in CMSII; and (b) complex I is necessary to avoid redox disruption of photosynthesis in conditions where leaf mitochondria must oxidize both respiratory and photorespiratory substrates simultaneously.
Figures



References
-
- Backhausen JE, Emmerlich A, Holtgrefe S, Horton P, Nast G, Rogers JM, Müller-Röber B, Scheibe R. Transgenic potato plants with altered expression levels of chloroplast NADP-malate dehydrogenase: interactions between photosynthetic electron transport and malate metabolism in leaves and in isolated intact chloroplasts. Planta. 1998;207:105–114.
-
- Backhausen JE, Scheibe R. Adaptation of tobacco plants to elevated CO2: influence of leaf age on changes in physiology, redox states and NADP-malate dehydrogenase activity. J Exp Bot. 1999;50:665–675.
-
- Cornic G, Briantais J-M. Partitioning of photosynthetic electron flow between CO2 and O2 reduction in a C3 leaf (Phaseolus vulgaris L.) at different CO2 concentrations and during drought stress. Planta. 1991;183:178–184. - PubMed
-
- Douce R, Neuburger M. The uniqueness of plant mitochondria. Annu Rev Plant Physiol Plant Mol Biol. 1989;40:371–414.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

