Sugar-regulated expression of a putative hexose transport gene in grape
- PMID: 12529540
- PMCID: PMC166812
- DOI: 10.1104/pp.009522
Sugar-regulated expression of a putative hexose transport gene in grape
Abstract
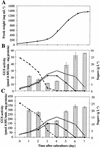
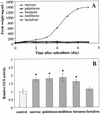
Different lengths of the promoter of grape (Vitis vinifera) VvHT1 (Hexose Transporter 1) gene, which encodes a putative hexose transporter expressed during the ripening of grape, have been transcriptionally fused to the beta-glucuronidase reporter gene. In transgenic tobacco (Nicotiana tabacum) transformed with these constructs, VvHT1 promoters were clearly responsible for the sink organ preferential expression. The potential sugar effectors of VvHT1 promoter were studied in tobacco cv Bright-Yellow 2 cells transformed with chimeric constructs. Glucose (56 mM), sucrose (Suc; 58 mM), and the non-transported Suc isomer palatinose doubled the beta-glucuronidase activity conferred by the VvHT1 promoter, whereas fructose did not affect it. These effects were the strongest with the 2.4-kb promoter, which contains all putative sugar-responsive elements (activating and repressing), but they were also significant with the 0.3-kb promoter, which contains only activating sugar boxes. The induction of VvHT1 expression by both Suc and palatinose was confirmed in the homologous grape berry cell culture. The data provide the first example of a putative sugar transporter, which is induced by both glucose and Suc in higher plants. Although induction of VvHT1 expression by Suc does not require transport, the presence of glucosyl moiety is necessary for Suc sensing. These results provide new insights into sugar sensing and signaling in plants.
Figures
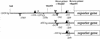





References
-
- Bearden JC. Quantitation of submicrogram quantities of protein by an improved protein-dye binding assay. Biochim Biophys Acta. 1978;533:525–529. - PubMed
-
- Borisjuk L, Walenta S, Weber H, Mueller-Klieser W, Wobus U. High-resolution histographical mapping of glucose concentrations in developing cotyledons of Vicia faba in relation to mitotic activity and storage processes: glucose as a possible developmental trigger. Plant J. 1998;15:583–591.
-
- Büttner M, Sauer N. Monosaccharide transporters in plants: structure, function and physiology. Biochim Biophys Acta. 2000;1465:263–274. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

