Characterization of the stringent response and rel(Bbu) expression in Borrelia burgdorferi
- PMID: 12533471
- PMCID: PMC142832
- DOI: 10.1128/JB.185.3.957-965.2003
Characterization of the stringent response and rel(Bbu) expression in Borrelia burgdorferi
Abstract
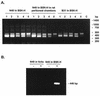
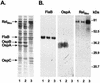
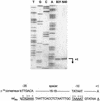
The stringent response is a global bacterial response to nutritional stress mediated by (p)ppGpp. We previously found that both noninfectious Borrelia burgdorferi strain B31 and infectious B. burgdorferi strain N40 produced large amounts of (p)ppGpp during growth in BSK-H medium and suggested that the stringent response was triggered in B. burgdorferi under these conditions. Here we report that (p)ppGpp levels in B. burgdorferi growing in BSK-II or BSK-H medium are not further increased by nutrient limitation or by serine hydroxamate-induced inhibition of protein synthesis and that the presence of (p)ppGpp during growth of N40 in BSK-H medium is not associated with decreased 16S rRNA synthesis. Decreased 16S rRNA synthesis was associated with the decreased growth rate of N40 seen during coculture with tick cells, which are growth conditions that were previously shown to decrease (p)ppGpp levels. One-half as much of the mRNA of the gene encoding the Rel protein of B. burgdorferi (rel(Bbu)) was produced by B31 as by N40 during in vitro growth (2 +/- 0.5 and 4 +/- 0.8 fg of rel(Bbu) mRNA/ng of total Borrelia RNA, respectively). Although the amounts of N40 rel(Bbu) mRNA were identical during growth in vitro and in rat peritoneal chambers, they were markedly decreased during growth in nymphal ticks. In contrast to the lack of change in rel(Bbu) mRNA levels, larger amounts of a 78-kDa protein that was cross-reactive with antibodies to Bacillus subtilis Rel(Bsu) were detected in immunoblots of N40 lysates after growth in rat peritoneal chambers than after growth in vitro. Differences in the level of production of (p)ppGpp between B31 and N40 could not be explained by differences in rel(Bbu) promoters since identical transcriptional start sites 309 nucleotides upstream from the B31 and N40 rel(Bbu) ATG start codon and identical sigma(70)-like promoters were identified by primer extension and sequencing analysis. rel(Bbu) complemented an Escherichia coli CF1693 relA spoT double mutant for growth on M9 minimal medium, and the transformed cells produced rel(Bbu) mRNA. These results indicate that rel(Bbu) is functional and that its transcription and translation and production of (p)ppGpp are affected by environmental conditions in strains N40 and B31. They also suggest that in B. burgdorferi, an organism with few rRNA operons that grows slowly, the role of (p)ppGpp may differ from the classic role played by this molecule in E. coli and that (p)ppGpp may not be responsible for growth rate control.
Figures



References
-
- Alban, P. S., P. W. Johnson, and D. R. Nelson. 2000. Serum-starvation-induced changes in protein synthesis and morphology of Borrelia burgdorferi. Microbiology 146:119-127. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

