Rhamnolipid surfactant production affects biofilm architecture in Pseudomonas aeruginosa PAO1
- PMID: 12533479
- PMCID: PMC142794
- DOI: 10.1128/JB.185.3.1027-1036.2003
Rhamnolipid surfactant production affects biofilm architecture in Pseudomonas aeruginosa PAO1
Abstract
In response to certain environmental signals, bacteria will differentiate from an independent free-living mode of growth and take up an interdependent surface-attached existence. These surface-attached microbial communities are known as biofilms. In flowing systems where nutrients are available, biofilms can develop into elaborate three-dimensional structures. The development of biofilm architecture, particularly the spatial arrangement of colonies within the matrix and the open areas surrounding the colonies, is thought to be fundamental to the function of these complex communities. Here we report a new role for rhamnolipid surfactants produced by the opportunistic pathogen Pseudomonas aeruginosa in the maintenance of biofilm architecture. Biofilms produced by mutants deficient in rhamnolipid synthesis do not maintain the noncolonized channels surrounding macrocolonies. We provide evidence that surfactants may be able to maintain open channels by affecting cell-cell interactions and the attachment of bacterial cells to surfaces. The induced synthesis of rhamnolipids during the later stages of biofilm development (when cell density is high) implies an active mechanism whereby the bacteria exploit intercellular interaction and communication to actively maintain these channels. We propose that the maintenance of biofilm architecture represents a previously unrecognized step in the development of these microbial communities.
Figures
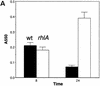

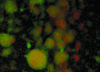
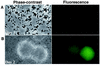
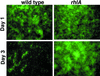

Comment in
-
Resident parking only: rhamnolipids maintain fluid channels in biofilms.J Bacteriol. 2003 Feb;185(3):699-700. doi: 10.1128/JB.185.3.699-700.2003. J Bacteriol. 2003. PMID: 12533444 Free PMC article. No abstract available.
References
-
- Christensen, B. B., C. Sternberg, J. B. Andersen, R. J. Palmer, Jr., A. T. Nielsen, M. Givskov, and S. Molin. 1999. Molecular tools for study of biofilm physiology. Methods Enzymol. 310:20-42. - PubMed
-
- Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:318-322. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

