Characterization of the CipA scaffolding protein and in vivo production of a minicellulosome in Clostridium acetobutylicum
- PMID: 12533485
- PMCID: PMC142813
- DOI: 10.1128/JB.185.3.1092-1096.2003
Characterization of the CipA scaffolding protein and in vivo production of a minicellulosome in Clostridium acetobutylicum
Abstract
The cipA gene encoding the Clostridium acetobutylicum scaffolding protein CipA was cloned and expressed in Escherichia coli. CipA contains an N-terminal signal peptide, a family 3a cellulose-binding domain (CBD), five type I cohesin domains, and six hydrophilic domains. The uniqueness of CipA lies in the enchainment of cohesin domains that are all separated by a hydrophilic domain. Affinity-purified CipA was used in equilibrium-binding experiments to characterize the interaction of CipA with crystalline cellulose. A K(d) of 0.038 micro M and a [C](max) of 0.43 micro mol of CipA bound per g of Avicel were determined. A mini-CipA polypeptide consisting of a CBD3a and two cohesin domains was overexpressed in C. acetobutylicum, yielding the in vivo formation of a minicellulosome. This is to our knowledge the first demonstration of the in vivo assembly of a recombinant minicellulosome.
Figures

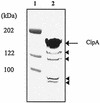

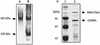
Comment in
-
Microbial conversion of corn stalks to riches.J Bacteriol. 2003 Feb;185(3):701-2. doi: 10.1128/JB.185.3.701-702.2003. J Bacteriol. 2003. PMID: 12533445 Free PMC article. No abstract available.
References
-
- Ciruela, A., H. J. Gilbert, B. R. S. Ali, and G. P. Hazlewood. 1998. Synergistic interaction of the cellulosome integrating protein (CipA) from Clostridium thermocellum with a cellulosomal endoglucanase. FEBS Lett. 422:221-224. - PubMed
-
- Doi, R. H., J. S. Park, C. C. Liu, L. M. Malburg, Jr., Y. Tamaru, A. Ichiishi, and A. Ibrahim. 1998. Cellulosome and noncellulosomal cellulases of Clostridium cellulovorans. Extremophiles 2:53-60. - PubMed
-
- Lamed, R., R. Kenig, E. Morag, S. Yaron, Y. Shoham, and E. A. Bayer. 2001. Nonproteolytic cleavage of aspartyl proline bonds in the cellulosomal scaffoldin subunit from Clostridium thermocellum. Appl. Biochem. Biotechnol. 90:67-73. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

