Genetic and biochemical studies of phosphatase activity of PhoR
- PMID: 12533489
- PMCID: PMC142828
- DOI: 10.1128/JB.185.3.1112-1115.2003
Genetic and biochemical studies of phosphatase activity of PhoR
Abstract
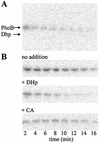
In Escherichia coli, PhoR is the histidine kinase of the phosphate regulon. It has been postulated that PhoR may function as a phospho-PhoB phosphatase. Experiments with four precise phoR deletion mutants supported this hypothesis and suggested that this activity resides within the histidine phosphorylation domain. This biochemical activity was confirmed by using a separately expressed histidine phosphorylation domain.
Figures


References
-
- Bird, T. H., S. Du, and C. E. Bauer. 1999. Autophosphorylation, phosphotransfer, and DNA-binding properties of the RegB/RegA two-component regulatory system in Rhodobacter capsulatus. J. Biol. Chem. 274:16343-16348. - PubMed
-
- Dutta, R., L. Qin, and M. Inouye. 1999. Histidine kinases: diversity of domain organization. Mol. Microbiol. 34:633-640. - PubMed
-
- Hoch, J. A., and T. J. Silhavy. 1995. Two-component signal transduction. American Society for Microbiology, Washington, D.C.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

