Tbx1 is regulated by tissue-specific forkhead proteins through a common Sonic hedgehog-responsive enhancer
- PMID: 12533514
- PMCID: PMC195981
- DOI: 10.1101/gad.1048903
Tbx1 is regulated by tissue-specific forkhead proteins through a common Sonic hedgehog-responsive enhancer
Abstract
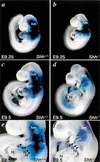
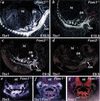
Haploinsufficiency of Tbx1 is likely a major determinant of cardiac and craniofacial birth defects associated with DiGeorge syndrome. Although mice deficient in Tbx1 exhibit pharyngeal and aortic arch defects, the developmental program and mechanisms through which Tbx1 functions are relatively unknown. We identified a single cis-element upstream of Tbx1 that recognized winged helix/forkhead box (Fox)-containing transcription factors and was essential for regulation of Tbx1 transcription in the pharyngeal endoderm and head mesenchyme. The Tbx1 regulatory region was responsive to signaling by Sonic hedgehog (Shh) in vivo. We show that Shh is necessary for aortic arch development, similar to Tbx1, and is also required for expression of Foxa2 and Foxc2 in the pharyngeal endoderm and head mesenchyme, respectively. Foxa2, Foxc1, or Foxc2 could bind and activate transcription through the critical cis-element upstream of Tbx1, and Foxc proteins were required, within their expression domains, for Tbx1 transcription in vivo. We propose that Tbx1 is a direct transcriptional target of Fox proteins and that Fox proteins may serve an intermediary role in Shh regulation of Tbx1.
Figures





References
-
- Abu-Issa R, Smyth G, Smoak I, Yamamura KI, Meyers EN. Fgf8 is required for pharyngeal arch and cardiovascular development in the mouse. Development. 2002;129:4613–4625. - PubMed
-
- Ang SL, Rossant J. HNF-3 β is essential for node and notochord formation in mouse development. Cell. 1994;78:561–574. - PubMed
-
- Bollag RJ, Siegfried Z, Cebra-Thomas JA, Garvey N, Davison EM, Silver LM. An ancient family of embryonically expressed mouse genes sharing a conserved protein motif with the T locus. Nat Genet. 1994;7:383–389. - PubMed
-
- Chapman DL, Garvey N, Hancock S, Alexiou M, Agulnik SI, Gibson-Brown JJ, Cebra-Thomas J, Bollag RJ, Silver LM, Papaioannou VE. Expression of the T-box family genes, Tbx1–Tbx5, during early mouse development. Dev Dyn. 1996;206:379–390. - PubMed
-
- Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–413. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
