Persistent changes in spontaneous firing of Purkinje neurons triggered by the nitric oxide signaling cascade
- PMID: 12533595
- PMCID: PMC6741890
- DOI: 10.1523/JNEUROSCI.23-02-00367.2003
Persistent changes in spontaneous firing of Purkinje neurons triggered by the nitric oxide signaling cascade
Abstract
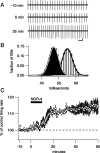
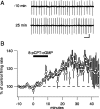
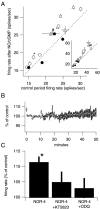
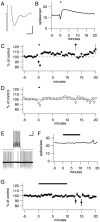
Many types of neurons fire spontaneously because of the activity of pacemaking ion channels. Although endogenous firing can serve as a persistent signal to downstream targets, little attention has been paid to factors that might modulate such intrinsic electrical activity. We tested for modulation of spontaneous firing of Purkinje neurons in cerebellar slices under conditions in which principal synaptic inputs were blocked. Loose-patch recordings from single neurons show that sustained (>40 min) increases in the spontaneous firing rate can be triggered by activation of the nitric oxide-cGMP signaling pathway. Inhibitors of soluble guanylate cyclase and protein kinase G block this modulation. Increases in firing rate are also observed after stimulation of parallel fibers but not in response to basket cell activity. These findings elucidate a novel role for the nitric oxide-cGMP signaling cascade in the brain. This mechanism could permit long-term adjustments in the baseline firing rate of endogenously active neurons in response to changes in afferent activity.
Figures
Similar articles
-
On the role of nitric oxide in hippocampal long-term potentiation.J Neurosci. 2003 Mar 1;23(5):1941-8. doi: 10.1523/JNEUROSCI.23-05-01941.2003. J Neurosci. 2003. PMID: 12629199 Free PMC article.
-
Nitric oxide as intracellular modulator: internal production of NO increases neuronal excitability via modulation of several ionic conductances.Eur J Neurosci. 2012 Nov;36(10):3333-43. doi: 10.1111/j.1460-9568.2012.08260.x. Epub 2012 Aug 23. Eur J Neurosci. 2012. PMID: 22913584
-
Long-term potentiation in hippocampus involves sequential activation of soluble guanylate cyclase, cGMP-dependent protein kinase, and cGMP-degrading phosphodiesterase.J Neurosci. 2002 Dec 1;22(23):10116-22. doi: 10.1523/JNEUROSCI.22-23-10116.2002. J Neurosci. 2002. PMID: 12451112 Free PMC article.
-
Sequential activation of soluble guanylate cyclase, protein kinase G and cGMP-degrading phosphodiesterase is necessary for proper induction of long-term potentiation in CA1 of hippocampus. Alterations in hyperammonemia.Neurochem Int. 2004 Nov;45(6):895-901. doi: 10.1016/j.neuint.2004.03.020. Neurochem Int. 2004. PMID: 15312984 Review.
-
Brain regional alterations in the modulation of the glutamate-nitric oxide-cGMP pathway in liver cirrhosis. Role of hyperammonemia and cell types involved.Neurochem Int. 2006 May-Jun;48(6-7):472-7. doi: 10.1016/j.neuint.2005.10.014. Epub 2006 Mar 6. Neurochem Int. 2006. PMID: 16517021 Review.
Cited by
-
A positive feedback loop linking enhanced mGluR function and basal calcium in spinocerebellar ataxia type 2.Elife. 2017 May 18;6:e26377. doi: 10.7554/eLife.26377. Elife. 2017. PMID: 28518055 Free PMC article.
-
Could respiration-driven blood oxygen changes modulate neural activity?Pflugers Arch. 2023 Jan;475(1):37-48. doi: 10.1007/s00424-022-02721-8. Epub 2022 Jun 28. Pflugers Arch. 2023. PMID: 35761104 Free PMC article. Review.
-
Protracted withdrawal from alcohol and drugs of abuse impairs long-term potentiation of intrinsic excitability in the juxtacapsular bed nucleus of the stria terminalis.J Neurosci. 2009 Apr 29;29(17):5389-401. doi: 10.1523/JNEUROSCI.5129-08.2009. J Neurosci. 2009. PMID: 19403807 Free PMC article.
-
The beat goes on: spontaneous firing in mammalian neuronal microcircuits.J Neurosci. 2004 Oct 20;24(42):9215-9. doi: 10.1523/JNEUROSCI.3375-04.2004. J Neurosci. 2004. PMID: 15496653 Free PMC article. Review. No abstract available.
-
The Emerging Concept of Intrinsic Plasticity: Activity-dependent Modulation of Intrinsic Excitability in Cerebellar Purkinje Cells and Motor Learning.Exp Neurobiol. 2018 Jun;27(3):139-154. doi: 10.5607/en.2018.27.3.139. Epub 2018 Jun 30. Exp Neurobiol. 2018. PMID: 30022866 Free PMC article. Review.
References
-
- Ahern GP, Hsu SF, Klyachko VA, Jackson MB. Induction of persistent sodium current by exogenous and endogenous nitric oxide. J Biol Chem. 2000;275:28810–28815. - PubMed
-
- Alioua A, Tanaka Y, Wallner M, Hofmann F, Ruth P, Meera P, Toro L. The large conductance, voltage-dependent, and calcium-sensitive K+ channel, Hslo, is a target of cGMP-dependent protein kinase phosphorylation in vivo. J Biol Chem. 1998;273:32950–32956. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
