Aberrant patterning of neuromuscular synapses in choline acetyltransferase-deficient mice
- PMID: 12533614
- PMCID: PMC6741871
- DOI: 10.1523/JNEUROSCI.23-02-00539.2003
Aberrant patterning of neuromuscular synapses in choline acetyltransferase-deficient mice
Abstract
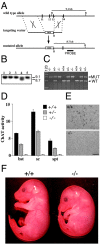
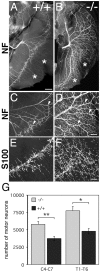
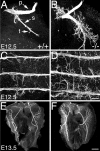
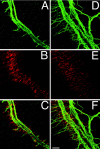
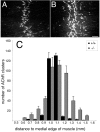
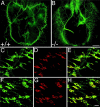
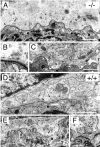
In this study we examined the developmental roles of acetylcholine (ACh) by establishing and analyzing mice lacking choline acetyltransferase (ChAT), the biosynthetic enzyme for ACh. As predicted, ChAT-deficient embryos lack both spontaneous and nerve-evoked postsynaptic potentials in muscle and die at birth. In mutant embryos, abnormally increased nerve branching occurs on contact with muscle, and hyperinnervation continues throughout subsequent prenatal development. Postsynaptically, ACh receptor clusters are markedly increased in number and occupy a broader muscle territory in the mutants. Concomitantly, the mutants have significantly more motor neurons than normal. At an ultrastructural level, nerve terminals are smaller in mutant neuromuscular junctions, and they make fewer synaptic contacts to the postsynaptic muscle membrane, although all of the typical synaptic components are present in the mutant. These results indicate that ChAT is uniquely essential for the patterning and formation of mammalian neuromuscular synapses.
Figures


References
-
- Belhage B, Hansen G, Elster L, Schousboe A. Effects of γ-aminobutyric acid (GABA) on synaptogenesis and synaptic function. Perspect Dev Neurobiol. 1998;5:235–246. - PubMed
-
- Braithwaite AW, Harris AJ. Neural influence on acetylcholine receptor clusters in embryonic development of skeletal muscles. Nature. 1979;279:549–551. - PubMed
-
- Brandon EP, Lin W, D'Amour KA, Pizzo DP, Dominguez B, Thode S, Thal LJ, Lee KF, Gage FH. Choline acetyltransferase knockout mice have defects in neuromuscular development. Soc Neurosci Abstr. 2000;26:411.8.
-
- Brockes JP. Assays for cholinergic properties in cultured rat Schwann cells. Proc R Soc Lond B Biol Sci. 1984;222:121–134. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
