Population pharmacokinetics of theophylline during paediatric extracorporeal membrane oxygenation
- PMID: 12534637
- PMCID: PMC1884193
- DOI: 10.1046/j.1365-2125.2003.01735.x
Population pharmacokinetics of theophylline during paediatric extracorporeal membrane oxygenation
Abstract
Aims: To determine the population pharmacokinetics of theophylline during extracorporeal membrane oxygenation (ECMO) from routine monitoring data.
Methods: Retrospective data were collected from 75 term neonates and children (age range 2 days to 17 years) receiving continuous infusions of aminophylline (mean rate 9.2 +/- 2.6 micro g kg-1 min-1) during ECMO. A total of 160 plasma concentrations (range 1-8 per patient), sampled at time intervals ranging from 10 h to 432 h, were included. Population PK analysis and model building were carried out using WinNonMix Professional (Version 2.0.1). Cross-validation was used to evaluate the validity and predictive accuracy of the model.
Results: A one-compartment model with first order elimination combined with an additive error model was found to best describe the data. Of the covariables tested, bodyweight significantly influenced clearance and volume of distribution, whereas age was an important determinant of clearance, as adjudged by the differences in the -2 x log likelihood (P < 0.005) and the residual error value. The final model parameters were estimated as: clearance (l h-1) = 0.023 x bodyweight (kg) + 0.000057 x age (days) and volume of distribution (l) = 0.57 x bodyweight (kg). The interindividual variability in clearance and volume of distribution was 38% and 40%, respectively. The residual error corresponded to a standard deviation of 3.6 mg l-1. Cross-validation revealed a median (95% confidence interval) model bias of 9.4% (2.9, 16.5%) and precision of 29.5% (24.8, 36.0%).
Conclusions: The estimated clearance is significantly lower, and volume of distribution higher, than previously reported in non-ECMO patients of similar age. These differences are probably a result of the expanded circulating volume during ECMO and altered renal and hepatic physiology in this critically ill group. Large interindividual variability reflects the heterogeneous nature of patients treated on ECMO.
Figures
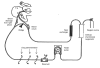
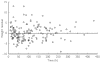
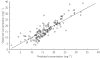
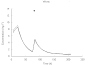

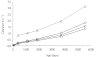
 ) percentile weights) and a paediatric population study by Driscoll et al.[12] (▵)
) percentile weights) and a paediatric population study by Driscoll et al.[12] (▵)
References
-
- Sigurd B, Olesen K. The supra-additive natriuretic effect addition of theophylline ethylenediamine and bumetanide in congestive heart failure. Am Heart J. 1977;94:168–174. - PubMed
-
- Lochan S, Adeniyi-Jones S, Assadi F, Frey B, Marcus S, Baumgart S. Coadministration of theophylline enhances diuretic response to furosemide in infants during extracorporeal membrane oxygenation. A randomized controlled pilot study. J Pediatr. 1998;133:86–89. - PubMed
-
- Pretzlaff R, Vardis R, Pollack M. Aminophylline in the treatment of fluid overload. Crit Care Med. 1999;27:2782–2785. - PubMed
-
- Nassif E, Weinberger M, Shannon D, Guiang S, Hendeles L, Jimenez D, et al. Theophylline disposition in infancy. J Pediatrics. 1981;98:158–161. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

