Tolerability and efficacy of single dose albendazole, diethylcarbamazine citrate (DEC) or co-administration of albendazole with DEC in the clearance of Wuchereria bancrofti in asymptomatic microfilaraemic volunteers in Pondicherry, South India: a hospital-based study
- PMID: 12537598
- PMCID: PMC139957
- DOI: 10.1186/1475-2883-1-1
Tolerability and efficacy of single dose albendazole, diethylcarbamazine citrate (DEC) or co-administration of albendazole with DEC in the clearance of Wuchereria bancrofti in asymptomatic microfilaraemic volunteers in Pondicherry, South India: a hospital-based study
Abstract
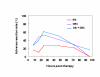
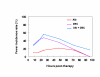
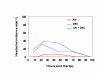
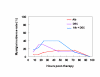
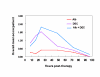
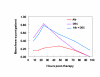
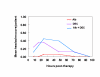
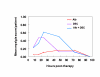
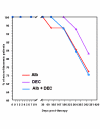
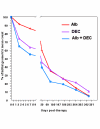
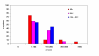
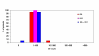
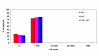
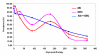
BACKGROUND: The tolerability and efficacy of single dose albendazole (400 mg), diethylcarbamazine citrate (DEC) (6 mg/kg bodyweight) or co-administration of albendazole (400 mg) + DEC (6 mg/kg bodyweight) was studied in 54 asymptomatic Wuchereria bancrofti microfilaraemic volunteers in a double blind hospital-based clinical study. RESULTS: There was no significant difference in the overall incidence of adverse reactions between the three drug groups [42.1% (albendazole), 52.9% (DEC) and 61.1% (albendazole + DEC); P > 0.05]. The mean score of adverse reaction intensity did not differ significantly between the DEC and albendazole + DEC groups. However, the values in these two groups were significantly higher compared to that of albendazole alone [1.8 +/- 3.0 (albendazole) vs. 5.6 +/- 7.1 (DEC), 6.7 +/- 6.6 (albendazole + DEC); P < 0.05]. By day 360 post-therapy there was no significant difference between the three drug groups in relation to the clearance of microfilaria [26.3% (albendazole), 17.6% (DEC), 27.8% (albendazole + DEC)], reduction in geometric mean parasite density [94.7% (albendazole), 89.5% (DEC), 95.4% (albendazole + DEC)] or reduction in filarial antigenaemia [83% (albendazole), 87% (DEC), 75% (albendazole + DEC)]. Furthermore, there was a significant decrease in mean geometric parasite density (P < 0.05) as well as antigenaemia optical density values (P < 0.01) between pre-therapy levels and day 360 post-therapy in all three groups. CONCLUSIONS: This study has shown that single dose albendazole (400 mg) has similar efficacy in the clearance of microfilaria as that of DEC or the co-administration of the two drugs. The results strengthen the rationale of using albendazole for mass annual single dose administration for the control of transmission of lymphatic filariasis.
Figures
References
-
- Sabesan S, Palaniyandi M, Das PK, Michael E. Mapping of lymphatic filariasis in India. Ann Trop Med Parasitol. 2000;94:591–606. - PubMed
-
- Das PK, Pani SP. "Filariasis", Epidemiology and control. In: Prof ML Sood, editor. Helminthology in India. 2002.
-
- Das PK, Pani SP. Towards elimination of lymphatic filariasis in India: Problems, challenges, opportunities and new initiatives. J Int Med Sci Acad. 2000;13:18–26.
LinkOut - more resources
Full Text Sources
Research Materials

