Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective Th1 responses to herpes simplex virus-2
- PMID: 12538655
- PMCID: PMC2193810
- DOI: 10.1084/jem.20021109
Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective Th1 responses to herpes simplex virus-2
Abstract
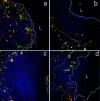
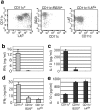
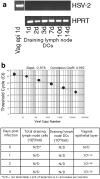
Herpes simplex virus (HSV) type 2 infection occurs primarily at the genital mucosal surfaces and is a leading cause of ulcerative lesions. Despite the availability of animal models for HSV-2 infection, little is known regarding the mechanism of immune induction within the vaginal mucosa. Here, we examined the cell types responsible for the initiation of protective Th1 immunity to HSV-2. Intravaginal inoculation of HSV-2 led to a rapid recruitment of submucosal dendritic cells (DCs) to the infected epithelium. Subsequently, CD11c(+) DCs harboring viral peptides in the context of MHC class II molecules emerged in the draining lymph nodes and were found to be responsible for the stimulation of IFNgamma secretion from HSV-specific CD4(+) T cells. Other antigen-presenting cells including B cells and macrophages did not present viral peptides to T cells in the draining lymph nodes. Next, we assessed the relative contribution to immune generation by the Langerhans cells in the vaginal epithelium, the submucosal CD11b(+) DCs, and the CD8alpha(+) lymph node DCs. Analysis of these DC populations from the draining lymph nodes revealed that only the CD11b(+) submucosal DCs, but not Langerhans cell-derived or CD8alpha(+) DCs, presented viral antigens to CD4(+) T cells and induced IFNgamma secretion. These results demonstrate a previously unanticipated role for submucosal DCs in the generation of protective Th1 immune responses to HSV-2 in the vaginal mucosa, and suggest their importance in immunity to other sexually transmitted diseases.
Figures




References
-
- Meunier, L., A. Gonzalez-Ramos, and K.D. Cooper. 1993. Heterogeneous populations of class II MHC+ cells in human dermal cell suspensions. Identification of a small subset responsible for potent dermal antigen-presenting cell activity with features analogous to Langerhans cells. J. Immunol. 151:4067–4080. - PubMed
-
- Baker, D.A., and S.A. Plotkin. 1978. Enhancement of vaginal infection in mice by herpes simplex virus type II with progesterone. Proc. Soc. Exp. Biol. Med. 158:131–134. - PubMed
-
- Gallichan, W.S., and K.L. Rosenthal. 1996. Effects of the estrous cycle on local humoral immune responses and protection of intranasally immunized female mice against herpes simplex virus type 2 infection in the genital tract. Virology. 224:487–497. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

