Differential regulation of cathepsin S and cathepsin L in interferon gamma-treated macrophages
- PMID: 12538657
- PMCID: PMC2193812
- DOI: 10.1084/jem.20020978
Differential regulation of cathepsin S and cathepsin L in interferon gamma-treated macrophages
Abstract
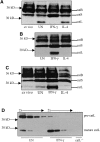
Cathepsin S (catS) and cathepsin L (catL) mediate late stages of invariant chain (Ii) degradation in discrete antigen-presenting cell types. Macrophages (Mphis) are unique in that they express both proteases and here we sought to determine the relative contribution of each enzyme. We observe that catL plays no significant role in Ii cleavage in interferon (IFN)-gamma-stimulated Mphis. In addition, our studies show that the level of catL activity is significantly decreased in Mphis cultured in the presence of IFN-gamma whereas catS activity increases. The decrease in catL activity upon cytokine treatment occurs despite the persistence of high levels of mature catL protein, suggesting that a specific inhibitor of the enzyme is up-regulated in IFN-gamma-stimulated peritoneal Mphis. Similar inhibition of activity is observed in dendritic cells engineered to overexpress catL. Such enzymatic inhibition in Mphis exhibits only partial dependence upon Ii and therefore, other mechanisms of catL inhibition are regulated by IFN-gamma. Thus, during a T helper cell type 1 immune response catL inhibition in Mphis results in preferential usage of catS, such that major histocompatibility complex class II presentation by all bone marrow-derived antigen-presenting cell is regulated by catS.
Figures


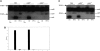



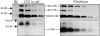
References
-
- Cresswell, P. 1994. Assembly, transport, and function of MHC class II molecules. Annu. Rev. Immunol. 12:259–293. - PubMed
-
- Cresswell, P. 1996. Invariant chain structure and MHC class II function. Cell. 84:505–507. - PubMed
-
- Bakke, O., and B. Dobberstein. 1990. MHC class II-associated invariant chain contains a sorting signal for endosomal compartments. Cell. 63:707–716. - PubMed
-
- Lotteau, V., L. Teyton, A. Peleraux, T. Nilsson, L. Karlsson, S.L. Schmid, V. Quaranta, and P.A. Peterson. 1990. Intracellular transport of class II MHC molecules directed by invariant chain. Nature. 348:600–605. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

