Host prostaglandin E(2)-EP3 signaling regulates tumor-associated angiogenesis and tumor growth
- PMID: 12538661
- PMCID: PMC2193807
- DOI: 10.1084/jem.20021408
Host prostaglandin E(2)-EP3 signaling regulates tumor-associated angiogenesis and tumor growth
Abstract
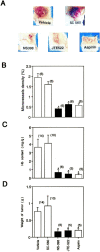
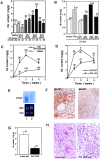
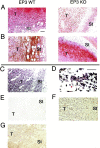
Nonsteroidal antiinflammatories are known to suppress incidence and progression of malignancies including colorectal cancers. However, the precise mechanism of this action remains unknown. Using prostaglandin (PG) receptor knockout mice, we have evaluated a role of PGs in tumor-associated angiogenesis and tumor growth, and identified PG receptors involved. Sarcoma-180 cells implanted in wild-type (WT) mice formed a tumor with extensive angiogenesis, which was greatly suppressed by specific inhibitors for cyclooxygenase (COX)-2 but not for COX-1. Angiogenesis in sponge implantation model, which can mimic tumor-stromal angiogenesis, was markedly suppressed in mice lacking EP3 (EP3(-/-)) with reduced expression of vascular endothelial growth factor (VEGF) around the sponge implants. Further, implanted tumor growth (sarcoma-180, Lewis lung carcinoma) was markedly suppressed in EP3(-/-), in which tumor-associated angiogenesis was also reduced. Immunohistochemical analysis revealed that major VEGF-expressing cells in the stroma were CD3/Mac-1 double-negative fibroblasts, and that VEGF-expression in the stroma was markedly reduced in EP3(-/-), compared with WT. Application of an EP3 receptor antagonist inhibited tumor growth and angiogenesis in WT, but not in EP3(-/-). These results demonstrate significance of host stromal PGE(2)-EP3 receptor signaling in tumor development and angiogenesis. An EP3 receptor antagonist may be a candidate of chemopreventive agents effective for malignant tumors.
Figures
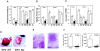

References
-
- Smalley, W., and R.N. Dubois. 1997. Colorectal cancer and non steroidal anti-inflammatory drugs. Adv. Pharmacol. 39:1–20. - PubMed
-
- Ohshima, M., J.E. Dinchuk, S.L. Kargman, H. Oshima, B. Hancock, E. Kwong, J.M. Trzaskos, J.F. Evans, and M.M. Taketo. 1996. Suppression of intestinal polyposis in Apc Δ716 knockout mice by inhibition of cyclooxygenase 2 (COX-2). Cell. 87:803–809. - PubMed
-
- Marx, J. 2001. Cancer research. Anti-inflammatories inhibit cancer growth–but how? Science. 291:581–582. - PubMed
-
- Prescott, S.M., and R.L. White. 1996. Self-promotion? Intimate connections between APC and prostaglandin H synthase-2. Cell. 87:783–786. - PubMed
-
- Shiff, S.J., and B. Rigas. 1997. Nonsteroidal anti-inflammatory drugs and colorectal cancer: evolving concepts of their chemopreventive actions. Gastroenterology. 113:1992–1998. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

