Normal tissue depresses while tumor tissue enhances human T cell responses in vivo to a novel self/tumor melanoma antigen, OA1
- PMID: 12538723
- PMCID: PMC2241741
- DOI: 10.4049/jimmunol.170.3.1579
Normal tissue depresses while tumor tissue enhances human T cell responses in vivo to a novel self/tumor melanoma antigen, OA1
Abstract
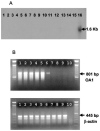
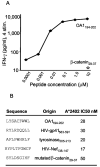
Antitumor T cells often recognize targets that are nonmutated "self" tissue differentiation Ags, but the relative impact of Ag expression by normal and transformed tissue for a human self/tumor Ag has not been studied. To examine the influence of self-tolerance mechanisms on the function of self/tumor-specific T cell responses in humans, we sought to identify an Ag that was expressed, processed, and presented in an MHC-restricted fashion by tumor cells, but for which there was the human equivalent of a "knockout." In this study, we report the first immunological characterization of a melanoma/melanocyte differentiation Ag, called OA1, which meets these criteria. This Ag, an X chromosome-encoded melanoma/melanocyte differentiation Ag, was completely deleted in a male patient. Using a newly identified HLA-A*2402-restricted epitope (LYSACFWWL) to study T cell tolerance, we found that OA1-specific T cell reactivity was more than five SD higher in the knockout patient that in normal controls. These data provide compelling evidence for T cell tolerance to OA1 in humans. Most surprisingly, we found elevated levels of OA1-specific T cells in patients with metastatic malignant melanoma, indicating that the tumor-bearing state partially reversed tolerance observed in normal (non-"knockout") individuals. Taken together, these findings indicated that tolerance can exist for self/tumor Ags in humans, and that this tolerance could be partially abrogated by the growth of the tumor, increasing the reactivity of tumor Ag-specific T cells. Thus, the tumor-bearing state reverses, in part, the tolerance of T cells that results from the normal expression of tissue differentiation Ags.
Figures


References
-
- Overwijk WW, Lee DS, Surman DR, Irvine KR, Touloukian CE, Chan CC, Carroll MW, Moss B, Rosenberg SA, Restifo NP. Vaccination with a recombinant vaccinia virus encoding a “self” antigen induces autoimmune vitiligo and tumor cell destruction in mice: requirement for CD4+ T lymphocytes. Proc. Natl. Acad. Sci. USA. 1999;96:2982. - PMC - PubMed
-
- Rosenberg SA. Progress in human tumour immunology and immuno-therapy. Nature. 2001;411:380. - PubMed
-
- Overwijk WW, Tsung A, Irvine KR, Parkhurst MR, Goletz TJ, Tsung K, Carroll MW, Liu C, Moss B, Rosenberg SA, Restifo NP. gp100/pmel 17 is a murine tumor rejection antigen: induction of “self”-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J. Exp. Med. 1998;188:277. - PMC - PubMed
-
- Mizoguchi H, O'Shea JJ, Longo DL, Loeffler CM, McVicar DW, Ochoa AC. Alterations in signal transduction molecules in T lymphocytes from tumor-bearing mice. Science. 1992;258:1795. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

