Oncogenic targeting of an activated tyrosine kinase to the Golgi apparatus in a glioblastoma
- PMID: 12538861
- PMCID: PMC298701
- DOI: 10.1073/pnas.242741799
Oncogenic targeting of an activated tyrosine kinase to the Golgi apparatus in a glioblastoma
Abstract
Activating oncogenic mutations of receptor tyrosine kinases (RTKs) have been reported in several types of cancers. In many cases, genomic rearrangements lead to the fusion of unrelated genes to the DNA coding for the kinase domain of RTKs. All RTK-derived fusion proteins reported so far display oligomerization sequences within the 5' fusion partners that are responsible for oncogenic activation. Here, we report a mechanism by which an altered RTK gains oncogenic potential in a glioblastoma cell line. A microdeletion on 6q21 results in the fusion of FIG, a gene coding for a Golgi apparatus-associated protein, to the kinase domain of the protooncogene c-ROS. The fused protein product FIG-ROS is a potent oncogene, and its transforming potential resides in its ability to interact with and become localized to the Golgi apparatus. Thus we have found a RTK fusion protein whose subcellular location leads to constitutive kinase activation and results in oncogenic transformation.
Figures
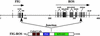




References
-
- Blume-Jensen P, Hunter T. Nature. 2001;411:355–365. - PubMed
-
- Lamorte L, Park M. Surg Oncol Clin North Am. 2001;10:271–288. , viii. - PubMed
-
- Sonnenberg-Riethmacher E, Walter B, Riethmacher D, Godecke S, Birchmeier C. Genes Dev. 1996;10:1184–1193. - PubMed
-
- Liu Z Z, Wada J, Kumar A, Carone F A, Takahashi M, Kanwar Y S. Dev Biol. 1996;178:133–148. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

