Adenosine receptor blockade reverses hypophagia and enhances locomotor activity of dopamine-deficient mice
- PMID: 12538862
- PMCID: PMC298775
- DOI: 10.1073/pnas.252753799
Adenosine receptor blockade reverses hypophagia and enhances locomotor activity of dopamine-deficient mice
Abstract
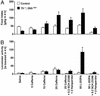
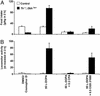
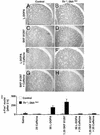
Adenosine receptors modulate dopaminergic function by regulating dopamine release in presynaptic neurons and intracellular signaling in postsynaptic striatal neurons. To investigate how adenosine impinges on the action of dopamine in feeding and locomotion, genetically altered, dopamine-deficient mice were treated with adenosine receptor antagonists. Acute administration of the nonselective adenosine receptor antagonist, caffeine (5-25 mgkg i.p.), reversed the hypophagia of mutant mice and induced hyperactivity in both control and mutant animals. However, caffeine treatment elicited much less hyperactivity in dopamine-deficient mice than did l-3,4-dihydroxyphenylalanine (l-dopa) administration, which partially restores dopamine content. Caffeine treatment enhanced feeding of l-dopa-treated mutants but, unexpectedly, it reduced their hyperlocomotion. Caffeine administration induced c-Fos expression in the cortex of dopamine-deficient mice but had no effect in the striatum by itself. Caffeine attenuated dopamine agonist-induced striatal c-Fos expression. An antagonist selective for adenosine A(2A) receptors induced feeding and locomotion in mutants much more effectively than an A(1) receptor antagonist. l-dopa-elicited feeding and hyperlocomotion were reduced in mutants treated with an A(1) receptor agonist, whereas an A(2A) receptor agonist decreased l-dopa-induced feeding without affecting locomotion. The observations suggest that the hypophagia and hypoactivity of mutants result not only because of the absence of dopamine but also because of the presence of A(2A) receptor signaling. This study of a genetic model of dopamine depletion provides evidence that A(2A) receptor antagonists could ameliorate the hypokinetic symptoms of advanced Parkinson's disease patients without inducing excessive motor activity.
Figures



Similar articles
-
Endogenous neurotensin attenuates dopamine-dependent locomotion and stereotypy.Brain Res. 2004 Oct 1;1022(1-2):71-80. doi: 10.1016/j.brainres.2004.06.061. Brain Res. 2004. PMID: 15353215
-
Cholecystokinin2 receptor-deficient mice display altered function of brain dopaminergic system.Psychopharmacology (Berl). 2001 Nov;158(2):198-204. doi: 10.1007/s002130100855. Psychopharmacology (Berl). 2001. PMID: 11702094
-
Adenosine receptor-mediated modulation of dopamine release in the nucleus accumbens depends on glutamate neurotransmission and N-methyl-D-aspartate receptor stimulation.J Neurochem. 2004 Nov;91(4):873-80. doi: 10.1111/j.1471-4159.2004.02761.x. J Neurochem. 2004. PMID: 15525341
-
DARPP chocolate: a caffeinated morsel of striatal signaling.Sci STKE. 2003 Jan 14;2003(165):PE2. doi: 10.1126/stke.2003.165.pe2. Sci STKE. 2003. PMID: 12527819 Review.
-
Adenosine and dopamine receptor interactions in striatum and caffeine-induced behavioral activation.Comp Med. 2007 Dec;57(6):538-45. Comp Med. 2007. PMID: 18246865 Review.
Cited by
-
Molecular and pharmacodynamic interactions between caffeine and dopaminergic system.J Med Life. 2014;7 Spec No. 4(Spec Iss 4):30-8. J Med Life. 2014. PMID: 27057246 Free PMC article. Review.
-
Restriction of dopamine signaling to the dorsolateral striatum is sufficient for many cognitive behaviors.Proc Natl Acad Sci U S A. 2009 Aug 25;106(34):14664-9. doi: 10.1073/pnas.0907299106. Epub 2009 Aug 10. Proc Natl Acad Sci U S A. 2009. PMID: 19667174 Free PMC article.
-
Adenosine-dopamine interactions revealed in knockout mice.J Mol Neurosci. 2005;26(2-3):239-44. doi: 10.1385/JMN:26:2-3:239. J Mol Neurosci. 2005. PMID: 16012197 Review.
-
Dopamine-independent locomotor actions of amphetamines in a novel acute mouse model of Parkinson disease.PLoS Biol. 2005 Aug;3(8):e271. doi: 10.1371/journal.pbio.0030271. Epub 2005 Aug 2. PLoS Biol. 2005. PMID: 16050778 Free PMC article.
-
Critical View on the Usage of Ribavirin in Already Existing Psychostimulant-Use Disorder.Curr Pharm Des. 2020;26(4):466-484. doi: 10.2174/1381612826666200115094642. Curr Pharm Des. 2020. PMID: 31939725 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

