Parallel changes in gene expression after 20,000 generations of evolution in Escherichiacoli
- PMID: 12538876
- PMCID: PMC298728
- DOI: 10.1073/pnas.0334340100
Parallel changes in gene expression after 20,000 generations of evolution in Escherichiacoli
Abstract
Twelve populations of Escherichia coli, derived from a common ancestor, evolved in a glucose-limited medium for 20,000 generations. Here we use DNA expression arrays to examine whether gene-expression profiles in two populations evolved in parallel, which would indicate adaptation, and to gain insight into the mechanisms underlying their adaptation. We compared the expression profile of the ancestor to that of clones sampled from both populations after 20,000 generations. The expression of 59 genes had changed significantly in both populations. Remarkably, all 59 were changed in the same direction relative to the ancestor. Many of these genes were members of the cAMP-cAMP receptor protein (CRP) and guanosine tetraphosphate (ppGpp) regulons. Sequencing of several genes controlling the effectors of these regulons found a nonsynonymous mutation in spoT in one population. Moving this mutation into the ancestral background showed that it increased fitness and produced many of the expression changes manifest after 20,000 generations. The same mutation had no effect on fitness when introduced into the other evolved population, indicating that a mutation of similar effect was present already. Our study demonstrates the utility of expression arrays for addressing evolutionary issues including the quantitative measurement of parallel evolution in independent lineages and the identification of beneficial mutations.
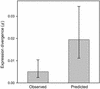
Figures


References
-
- Nosil P, Crespi B J, Sandoval C P. Nature. 2001;417:440–443. - PubMed
-
- Harvey P H, Pagel M. The Comparative Method in Evolutionary Biology. Oxford: Oxford Univ. Press; 1991.
-
- Simpson G G. The Major Features of Evolution. New York: Columbia Univ. Press; 1953.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

