Plasmodium falciparum infection elicits both variant-specific and cross-reactive antibodies against variant surface antigens
- PMID: 12540535
- PMCID: PMC145347
- DOI: 10.1128/IAI.71.2.597-604.2003
Plasmodium falciparum infection elicits both variant-specific and cross-reactive antibodies against variant surface antigens
Abstract
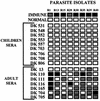
Naturally acquired antibodies to Plasmodium falciparum erythrocyte membrane protein-1 (PfEMP-1), the variant surface antigens expressed on the surface of infected erythrocytes, are thought to play a role in protection against P. falciparum malaria. Here, we have studied the development of antibodies to PfEMP-1 in adult malaria patients living in Rourkela, India, an area with a low malaria transmission rate, and prevalence of antibodies to PfEMP-1 in residents of San Dulakudar, India, a village in which P. falciparum malaria is hyperendemic. Convalescent-phase sera from adult malaria patients from Rourkela agglutinate homologous P. falciparum isolates as well as some heterologous isolates, suggesting that they develop partially cross-reactive antibodies to PfEMP-1 following infection. Adult sera from San Dulakudar agglutinate diverse P. falciparum isolates, suggesting that they have antibodies with wide recognition of diverse PfEMP-1. Mixed-agglutination assays using pairs of P. falciparum isolates confirm the presence of both variant-specific and partially cross-reactive antibodies in convalescent-phase sera from Rourkela and adult sera from San Dulakudar. Analysis of PfEMP-1 sequences suggests a molecular basis for the observed cross-reactivity.
Figures



Comment in
-
Understanding naturally acquired immunity to Plasmodium falciparum malaria.Infect Immun. 2003 Feb;71(2):589-90. doi: 10.1128/IAI.71.2.589-590.2003. Infect Immun. 2003. PMID: 12540533 Free PMC article. Review. No abstract available.
-
Specificity and cross-reactivity of Plasmodium falciparum variant surface antigen-specific antibody responses.Infect Immun. 2003 Apr;71(4):2296. doi: 10.1128/IAI.71.4.2296.2003. Infect Immun. 2003. PMID: 12654861 Free PMC article. No abstract available.
References
-
- Aguiar, J. C., G. R. Albrecht, P. Cegielski, B. M. Greenwood, J. B. Jensen, G. Lallinger, A. Martinez, I. A. McGregor, J. N. Minjas, J. Neequaye, M. E. Patarroyo, J. A. Sherwood, and R. J. Howard. 1992. Agglutination of Plasmodium falciparum-infected erythrocytes from east and west African isolates by human sera from distant geographic regions. Am. J. Trop. Med. Hyg. 47:621-632. - PubMed
-
- Baruch, D., B. Pasloske, H. Singh, B. Xiahui, X. Ma, M. Feldman, T. Taraschi, and R. J. Howard. 1995. Cloning of the Plasmodium falciparum gene encoding PfEMP1, a malarial variant antigen and cytoadherence receptor on the surface of parasitised human erythrocytes. Cell 82:77-87. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

