Photochemical gating of heterologous ion channels: remote control over genetically designated populations of neurons
- PMID: 12540832
- PMCID: PMC298776
- DOI: 10.1073/pnas.242738899
Photochemical gating of heterologous ion channels: remote control over genetically designated populations of neurons
Abstract
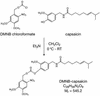
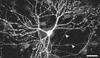
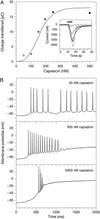
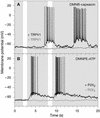
Heterologous proteins capable of transducing physical or chemical stimuli into electrical signals can be used to control the function of excitable cells in intact tissues or organisms. Restricted genetically to circumscribed populations of cellular targets, these selectively addressable sources of depolarizing current can supply distributed inputs to neural circuits, stimulate secretion, or regulate force and motility. In an initial demonstration of this principle, we have used elements of a G protein coupled signaling system, the phototransduction cascade of the fruit fly, to sensitize generalist vertebrate neurons to light [Zemelman, B. V., Lee, G. A., Ng, M. & Miesenböck, G. (2002) Neuron 33, 15-22]. We now describe the use of ectopically expressed ligand-gated ion channels as transducers of optical or pharmacological stimuli. When either the capsaicin receptor, TRPV1, the menthol receptor, TRPM8, or the ionotropic purinergic receptor P2X(2) was introduced into hippocampal neurons, the cells responded to pulsed applications of agonist with characteristic sequences of depolarization, spiking, and repolarization. Responses required cognate matches between receptor and agonist, peaked at firing frequencies of approximately 40 Hz, initiated and terminated rapidly, and did not attenuate. Precise dose-response relationships allowed current amplitudes and firing frequencies to be tuned by varying the concentration of ligand. Agonist could be administered either pharmacologically or, in the cases of TRPV1 and P2X(2), optically, through photorelease of the active compounds from the respective "caged" precursors, 4,5-dimethoxy-2-nitrobenzyl-capsaicin and P(3)-[1-(4,5-dimethoxy-2-nitrophenyl)ethyl]-ATP.
Figures
References
-
- Zemelman B V, Miesenböck G. Curr Opin Neurobiol. 2001;11:409–414. - PubMed
-
- Zemelman B V, Lee G A, Ng M, Miesenböck G. Neuron. 2002;33:15–22. - PubMed
-
- Hille B. Ion Channels of Excitable Membranes. Sunderland, MA: Sinauer; 2001.
-
- Stryer L. J Biol Chem. 1991;266:10711–10714. - PubMed
-
- Wickman K, Clapham D E. Physiol Rev. 1995;75:865–885. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

