Improved pharmacokinetics and reduced antibody reactivity of lysostaphin conjugated to polyethylene glycol
- PMID: 12543658
- PMCID: PMC151727
- DOI: 10.1128/AAC.47.2.554-558.2003
Improved pharmacokinetics and reduced antibody reactivity of lysostaphin conjugated to polyethylene glycol
Abstract
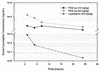
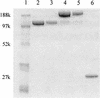
Lysostaphin is a 27-kDa endopeptidase that enzymatically disrupts the cell wall of Staphylococcus aureus and is a promising candidate for treating S. aureus blood-borne infections. It would be extremely useful to define conditions that would both increase lysostaphin's in vivo half-life to allow for more effective tissue distribution and reduce its immunogenicity. Conjugation of polyethylene glycol (PEG) to lysostaphin (PEGylation) was investigated as a means to accomplish these goals. Rather than using linear forms of PEG, branched PEGs were chosen as the initial candidates because their large spatial volumes prevent entry of the polymer into the enzyme's active sites, which could potentially reduce enzymatic function. Enzymatic activity for most PEGylated lysostaphins was reduced, but these compounds were still considerably active compared to unconjugated lysostaphin, with conjugates that had lower degrees of PEG modification having greater activity than those with higher degrees. PEGylated lysostaphin injected intravenously had a serum drug half-life of up to 24 h and resulted in much higher plasma drug concentrations than an equal dose of unconjugated lysostaphin, which had a half-life of less than 1 h. Finally, reduced binding affinity was shown for PEGylated lysostaphin in an antilysostaphin capture enzyme-linked immunosorbent assay, with some PEG-lysostaphin conjugates having binding affinities that were reduced more than 10-fold compared to unconjugated lysostaphin. These findings demonstrate that PEGylation of lysostaphin, while diminishing its S. aureus killing activity, results in prolonged serum drug persistence and reduced antibody binding. These features should significantly enhance lysostaphin's therapeutic value as an intravenous "antibiotic" against S. aureus.
Figures




Similar articles
-
Lysostaphin in treatment of neonatal Staphylococcus aureus infection.Antimicrob Agents Chemother. 2007 Jun;51(6):2198-200. doi: 10.1128/AAC.00506-06. Epub 2007 Apr 9. Antimicrob Agents Chemother. 2007. PMID: 17420212 Free PMC article.
-
[Site-specific PEGylation of recombinant lysostaphin].Sheng Wu Gong Cheng Xue Bao. 2016 Jan;32(1):127-34. Sheng Wu Gong Cheng Xue Bao. 2016. PMID: 27363205 Chinese.
-
Extended nasal residence time of lysostaphin and an anti-staphylococcal monoclonal antibody by delivery in semisolid or polymeric carriers.Pharm Res. 2004 Oct;21(10):1770-5. doi: 10.1023/b:pham.0000045227.16829.37. Pharm Res. 2004. PMID: 15553221
-
Lysostaphin: an antistaphylococcal agent.Appl Microbiol Biotechnol. 2008 Sep;80(4):555-61. doi: 10.1007/s00253-008-1579-y. Epub 2008 Jul 8. Appl Microbiol Biotechnol. 2008. PMID: 18607587 Review.
-
The impact of PEGylation on biological therapies.BioDrugs. 2008;22(5):315-29. doi: 10.2165/00063030-200822050-00004. BioDrugs. 2008. PMID: 18778113 Review.
Cited by
-
Engineering of Long-Circulating Peptidoglycan Hydrolases Enables Efficient Treatment of Systemic Staphylococcus aureus Infection.mBio. 2020 Sep 22;11(5):e01781-20. doi: 10.1128/mBio.01781-20. mBio. 2020. PMID: 32963004 Free PMC article.
-
Lysostaphin in treatment of neonatal Staphylococcus aureus infection.Antimicrob Agents Chemother. 2007 Jun;51(6):2198-200. doi: 10.1128/AAC.00506-06. Epub 2007 Apr 9. Antimicrob Agents Chemother. 2007. PMID: 17420212 Free PMC article.
-
microPET of tumor integrin alphavbeta3 expression using 18F-labeled PEGylated tetrameric RGD peptide (18F-FPRGD4).J Nucl Med. 2007 Sep;48(9):1536-44. doi: 10.2967/jnumed.107.040816. Epub 2007 Aug 17. J Nucl Med. 2007. PMID: 17704249 Free PMC article.
-
Deimmunized Lysostaphin Synergizes with Small-Molecule Chemotherapies and Resensitizes Methicillin-Resistant Staphylococcus aureus to β-Lactam Antibiotics.Antimicrob Agents Chemother. 2021 Feb 17;65(3):e01707-20. doi: 10.1128/AAC.01707-20. Print 2021 Feb 17. Antimicrob Agents Chemother. 2021. PMID: 33318001 Free PMC article.
-
Hydrogen/deuterium exchange mass spectrometry and site-directed disulfide cross-linking suggest an important dynamic interface between the two lysostaphin domains.Antimicrob Agents Chemother. 2013 Apr;57(4):1872-81. doi: 10.1128/AAC.02348-12. Epub 2013 Feb 4. Antimicrob Agents Chemother. 2013. PMID: 23380729 Free PMC article.
References
-
- Algranati, N. E., S. Sy, and M. Modi. 1999. A branched methoxy 40-kDa polyethylene glycol (PEG) moiety optimizes the pharmacokinetics of PEG-interferon alpha-2a (PEG-IFN) and may explain its enhanced efficacy in chronic hepatitis C. Hepatology 40(Suppl.):190A.
-
- Donohue, J. H., and S. A. Rosenberg. 1983. The fate of interleukin-2 after in vivo administration. J. Immunol. 130:2203-2208. - PubMed
-
- Francis, G. E., C. Delgado, and D. Fisher. 1992. PEG-modified proteins, p. 235-263. In M. C. Manning (ed.), Stability of protein pharmaceuticals: in vivo pathways of degradation and strategies for protein stabilization. Plenum Press, New York, N.Y.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

