Pharmacokinetics (PK), pharmacodynamics (PD), and PK-PD integration of danofloxacin in sheep biological fluids
- PMID: 12543670
- PMCID: PMC151775
- DOI: 10.1128/AAC.47.2.626-635.2003
Pharmacokinetics (PK), pharmacodynamics (PD), and PK-PD integration of danofloxacin in sheep biological fluids
Abstract
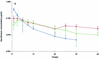
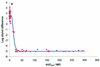
The fluoroquinolone antimicrobial drug danofloxacin was administered to sheep intravenously (i.v.) and intramuscularly (i.m.) at a dose of 1.25 mg/kg of body weight in a two-period crossover study. The pharmacokinetic properties of danofloxacin in serum, inflamed tissue cage fluid (exudate), and noninflamed tissue cage fluid (transudate) were established by using a tissue cage model. The in vitro and ex vivo activities of danofloxacin in serum, exudate, and transudate against a pathogenic strain of Mannheimia haemolytica were established. Integration of in vivo pharmacokinetic data with the in vitro MIC provided mean values for the area under the curve (AUC)/MIC for serum, exudate, and transudate of 60.5, 85.6, and 45.7 h, respectively, after i.v. dosing and 55.9, 77.9, and 49.1 h, respectively, after i.m. dosing. After i.m. dosing, the maximum concentration/MIC ratios for serum, exudate, and transudate were 10.8, 3.0, and 1.6, respectively. The ex vivo growth inhibition data after i.m. dosing were fitted to the inhibitory sigmoid E(max) equation to provide the values of AUC/MIC required to produce bacteriostasis, bactericidal activity, and elimination of bacteria. The respective values for serum were 17.8, 20.2, and 28.7 h, and slightly higher values were obtained for transudate and exudate. It is proposed that use of these data might provide a novel approach to the rational design of dosage schedules.
Figures






Similar articles
-
Pharmacokinetics and pharmacokinetic/pharmacodynamic integration of marbofloxacin in calf serum, exudate and transudate.J Vet Pharmacol Ther. 2002 Jun;25(3):161-74. doi: 10.1046/j.1365-2885.2002.00399.x. J Vet Pharmacol Ther. 2002. PMID: 12081611
-
PK-PD integration and modeling of marbofloxacin in sheep.Res Vet Sci. 2010 Feb;88(1):134-41. doi: 10.1016/j.rvsc.2009.05.013. Epub 2009 Jun 11. Res Vet Sci. 2010. PMID: 19523661 Clinical Trial.
-
Pharmacokinetic-pharmacodynamic integration of danofloxacin in the calf.Res Vet Sci. 2003 Jun;74(3):247-59. doi: 10.1016/s0034-5288(03)00005-5. Res Vet Sci. 2003. PMID: 12726744
-
Pharmacokinetic/pharmacodynamic relationships of antimicrobial drugs used in veterinary medicine.J Vet Pharmacol Ther. 2004 Dec;27(6):503-14. doi: 10.1111/j.1365-2885.2004.00603.x. J Vet Pharmacol Ther. 2004. PMID: 15601444 Review.
-
Mechanism-based pharmacokinetic-pharmacodynamic modeling of antimicrobial drug effects.J Pharmacokinet Pharmacodyn. 2007 Dec;34(6):727-51. doi: 10.1007/s10928-007-9069-x. Epub 2007 Sep 29. J Pharmacokinet Pharmacodyn. 2007. PMID: 17906920 Review.
Cited by
-
Pharmacokinetic-Pharmacodynamic Modeling to Study the Antipyretic Effect of Qingkailing Injection on Pyrexia Model Rats.Molecules. 2016 Mar 7;21(3):317. doi: 10.3390/molecules21030317. Molecules. 2016. PMID: 26959005 Free PMC article.
-
Comparative electrochemical study of veterinary drug danofloxacin at glassy carbon electrode and electrified liquid-liquid interface.Sci Rep. 2024 Jun 24;14(1):14489. doi: 10.1038/s41598-024-65246-3. Sci Rep. 2024. PMID: 38914687 Free PMC article.
-
Pharmacokinetic-pharmacodynamic modelling of danofloxacin in turkeys.Vet Res Commun. 2006 Oct;30(7):775-89. doi: 10.1007/s11259-006-3400-7. Vet Res Commun. 2006. PMID: 17004040 Clinical Trial.
-
Pharmacodynamic Parameters of Pharmacokinetic/Pharmacodynamic (PK/PD) Integration Models.Front Vet Sci. 2022 Mar 24;9:860472. doi: 10.3389/fvets.2022.860472. eCollection 2022. Front Vet Sci. 2022. PMID: 35400105 Free PMC article. Review.
-
Pharmacokinetics and pharmacodynamics integration of danofloxacin against Eschrichia coli in piglet ileum ultrafiltration probe model.Sci Rep. 2021 Jan 12;11(1):681. doi: 10.1038/s41598-020-80272-7. Sci Rep. 2021. PMID: 33436914 Free PMC article.
References
-
- Appelbaum, P. C., and P. A. Hunter. 2000. The fluoroquinolone antibacterials: past, present and future perspectives. Int. J. Antimicrob. Agents 16:5-15. - PubMed
-
- Blaser, J., B. B. Stone, M. C. Groner, and S. H. Zinner. 1987. Comparative study with enoxacin and netilmicin in a pharmacodynamic model to determine importance of ratio of antibiotic peak concentration to MIC for bacterial activity and emergence of resistance. Antimicrob. Agents Chemother. 31:1054-1060. - PMC - PubMed
-
- Cooper, A. C., J. R. Fuller, M. K. Fuller, P. Whittlestone, and D. R. Wise. 1993. In vitro activity of danofloxacin, tylosin and oxytetracycline against mycoplasmas of veterinary importance. Res. Vet. Sci. 54:329-334. - PubMed
-
- Czock, D., M., and M. Giehl. 1995. Aminoglycoside pharmacokinetics and dynamics: a nonlinear approach. Int. J. Clin. Pharmacol. Ther. 33:537-539. - PubMed
-
- Dalhoff, A., and U. Ullmann. 1990. Correlation between pharmacokinetics, pharmacodynamics and efficacy of antibacterial agents in animal models. Eur. J. Clin. Microbiol. Infect. Dis. 9:479-487. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

