Pharmacokinetics of florfenicol in healthy pigs and in pigs experimentally infected with Actinobacillus pleuropneumoniae
- PMID: 12543702
- PMCID: PMC151723
- DOI: 10.1128/AAC.47.2.820-823.2003
Pharmacokinetics of florfenicol in healthy pigs and in pigs experimentally infected with Actinobacillus pleuropneumoniae
Abstract
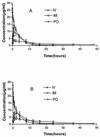
A comparative in vivo pharmacokinetic study of florfenicol was conducted in 18 crossbred pigs infected with Actinobacillus pleuropneumoniae following intravenous (i.v.), intramuscular (i.m.), or oral (p.o.) administration of a single dose of 20 mg/kg. The disease model was confirmed by clinical signs, X rays, pathohistologic examinations, and organism isolation. Florfenicol concentrations in plasma were determined by a validated high-performance liquid chromatography method with UV detection at a wavelength of 223 nm. Pharmacokinetic parameters were calculated by using the MCPKP software (Research Institute of Traditional Chinese Veterinary Medicine, Lanzhou, China). The disposition of florfenicol after a single i.v. bolus was described by a two-compartment model with values for the half-life at alpha phase (t(1/2alpha)), the half-life at beta phase (t(1/2beta)), the area under the concentration-time curve (AUC(0- infinity )), and the volume of distribution at steady state (V(ss)) of 0.37 h, 2.91 h, 64.86 micro g. h/ml, and 1.2 liter/kg, respectively. The concentration-time data fitted the one-compartment (after i.m.) and two-compartment (after p.o.) models with first-order absorption. The values for the maximum concentration of drug in serum (C(max)), t(1/2alpha), t(1/2beta), and bioavailability after i.m. and p.o. dosing were 4.00 and 8.11 micro g/ml, 0.12 and 3.91 h, 13.88 and 16.53 h, and 122.7 and 112.9%, respectively, for the two models. The study showed that florfenicol was absorbed quickly and completely, distributed widely, and eliminated slowly in the infected pigs, and there was no statistically significant difference between the pharmacokinetic profiles for the infected and healthy pigs.
Figures
References
-
- Adams, P. E., K. J. Varma, T. E. Powers, and J. F. Lamendola. 1987. Tissue concentrations and pharmacokinetics of florfenicol in male veal calves given repeated doses. Am. J. Vet. Res. 48:1725-1732. - PubMed
-
- Anonymous. 1999. Florfenicol (extension to pigs)—summary report, p. 1-4. In EMEA/MRL/591/99-FINAL. Committee for Veterinary Medicinal Products/The European Agency for the Evaluation of Medicinal Products. Cannary Wharf, London, United Kingdom.
-
- Barigazzi, G., P. Candotti, and E. Foni. 1996. In vitro susceptibility of 108 isolated Actinobacillus pleuropneumoniae strains to 17 antimicrobial agents from pig lungs in Italy in 1994-1995, p. 207. In Proceedings of the 14th International Pig Veterinary Society Congress, Bologna, Italy.
-
- Bretzlaff, K. N., C. A. Neff-Davis, R. S. Ott, G. D. Koritz, B. K. Gustafsson, and L. E. Davis. 1987. Florfenicol in non-lactating dairy cows: pharmacokinetics, binding to plasma proteins, and effects on phagocytosis by blood neutrophils. J. Vet. Pharmacol. Ther. 10:233-240. - PubMed
-
- Cannon, M., S. Harford, and J. Davies. 1990. A comparative study on the inhibitory actions of chloramphenicol, thiamphenicol and some fluorinated derivatives. J. Antimicrob. Chemother. 26:307-317. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical