Apolipoprotein B in cholesterol-containing drusen and basal deposits of human eyes with age-related maculopathy
- PMID: 12547700
- PMCID: PMC1851166
- DOI: 10.1016/S0002-9440(10)63836-9
Apolipoprotein B in cholesterol-containing drusen and basal deposits of human eyes with age-related maculopathy
Abstract
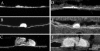
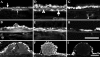
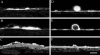
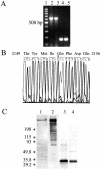
Lipids accumulate in Bruch's membrane (BrM), a specialized vascular intima of the eye, and in extracellular lesions associated with aging and age-related maculopathy (ARM). We tested the hypothesis that ARM and atherosclerotic cardiovascular disease share molecules and mechanisms pertaining to extracellular lipid accumulation by localizing cholesterol and apolipoprotein B (apo B) in BrM, basal deposits, and drusen. Human donor eyes were preserved <4 hours postmortem and cryosectioned. Sections were stained with traditional lipid stains and filipin for esterified and unesterified cholesterol or probed with antibodies to apo B, apo E, and apo C-III. Normal adult retinal pigment epithelium (RPE) was subjected to RT-PCR and Western blot analysis for apolipoprotein mRNA and protein. Esterified and unesterified cholesterol was present in all drusen and basal deposits of ARM and normal eyes. Both apo B and apo E but not apo C-III were found in BrM, drusen, and basal deposits. Fewer macular drusen were stained by traditional lipid stains and apolipoprotein antibodies than peripheral drusen. RPE contained apo B and apo E mRNA and protein. Finding cholesterol and apo B in sub-RPE deposits links ARM with important molecules and mechanisms in atherosclerosis initiation and progression. The combination of apo B mRNA and protein in RPE raises the possibility that intraocular assembly of apo B-containing lipoproteins is a pathway involved in forming cholesterol-enriched lesions in ARM.
Figures



References
-
- Klein R, Klein BEK, Linton KLP: Prevalence of age-related maculopathy. Ophthalmology 1992, 99:933-943 - PubMed
-
- Mitchell P, Smith W, Attebo K, Wang JJ: Prevalence of age-related maculopathy in Australia: The Blue Mountains Eye Study. Ophthalmology 1995, 102:1450-1460 - PubMed
-
- Vingerling JR, Dielemans I, Hofman A, Grobbee DE, Hijmering M, Kramer CFL, de Jong PTVM: The prevalence of age-related maculopathy in the Rotterdam study. Ophthalmology 1995, 102:205-210 - PubMed
-
- Green WR, Enger C: Age-related macular degeneration histopathologic studies: The 1992 Lorenz E. Zimmerman Lecture. Ophthalmology 1993, 100:1519-1535 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

