Transforming growth factor-beta mediates intestinal healing and susceptibility to injury in vitro and in vivo through epithelial cells
- PMID: 12547717
- PMCID: PMC1851153
- DOI: 10.1016/s0002-9440(10)63853-9
Transforming growth factor-beta mediates intestinal healing and susceptibility to injury in vitro and in vivo through epithelial cells
Abstract
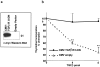
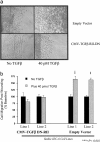
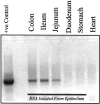
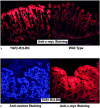
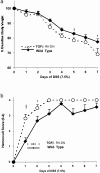
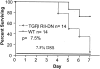
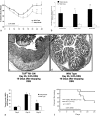
In vitro studies suggest that transforming growth factor (TGF)-beta has potent effects on gastrointestinal mucosal integrity, wound repair, and neoplasia. However, the multiplicity of actions of this peptide on many different cell types confounds efforts to define the role of TGF-beta within the intestinal epithelium in vivo. To delineate these effects selective blockade of intestinal epithelial TGF-beta activity was undertaken through targeted expression of a dominant-negative (DN) TGF-beta RII to intestinal epithelial cells in vitro and in vivo. Stable intestinal epithelial cell (IEC)-6 lines overexpressing TGF-beta RII-DN (nucleotides -7 to 573) were established. Transgenic mice overexpressing TGF-beta RII-DN under the regulation of a modified liver fatty acid-binding promoter (LFABP-PTS4) were constructed. In vitro healing was assessed by wounding of confluent monolayers. Colitis was induced by the addition of dextran sodium sulfate (2.5 to 7.5% w/v) to their drinking water. Overexpression of TGF-beta RII-DN in intestinal epithelial cell-6 cells resulted in a marked reduction in cell migration and TGF-beta-stimulated wound healing in vitro. TGF-beta RII-DN transgenic mice did not exhibit baseline intestinal inflammation or changes in survival, body weight, epithelial cell proliferation, aberrant crypt foci, or tumor formation. TGF-beta RII-DN mice were markedly more susceptible to dextran sodium sulfate-induced colitis and exhibited impaired recovery after colonic injury. TGF-beta is required for intestinal mucosal healing and TGF-beta modulation of the intestinal epithelium plays a central role in determining susceptibility to injury.
Figures

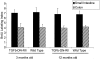

References
-
- Chen RH, Derynck R: Homomeric interactions between type II transforming growth factor-beta receptors. J Biol Chem 1994, 269:22868-22874 - PubMed
-
- Derynck R, Feng XH: TGF-beta receptor signaling. Biochim Biophys Acta 1997, 1333:F105-F150 - PubMed
-
- Zhao Y, Young SL: Requirement of transforming growth factor-beta (TGF-beta) type II receptor for TGF-beta-induced proliferation and growth inhibition. J Biol Chem 1996, 271:2369-2372 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

