Force measurements of the alpha5beta1 integrin-fibronectin interaction
- PMID: 12547805
- PMCID: PMC1302701
- DOI: 10.1016/S0006-3495(03)74940-6
Force measurements of the alpha5beta1 integrin-fibronectin interaction
Abstract
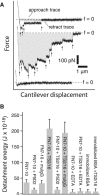
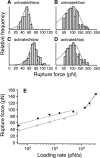
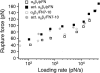
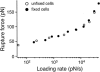
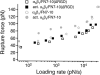
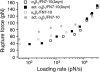
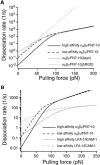
The interaction of the alpha(5)beta(1) integrin and its ligand, fibronectin (FN), plays a crucial role in the adhesion of cells to the extracellular matrix. An important intrinsic property of the alpha(5)beta(1)/FN interaction is the dynamic response of the complex to a pulling force. We have carried out atomic force microscopy measurements of the interaction between alpha(5)beta(1) and a fibronectin fragment derived from the seventh through tenth type III repeats of FN (i.e., FN7-10) containing both the arg-gly-asp (RGD) sequence and the synergy site. Direct force measurements obtained from an experimental system consisting of an alpha(5)beta(1) expressing K562 cell attached to the atomic force microscopy cantilever and FN7-10 adsorbed on a substrate were used to determine the dynamic response of the alpha(5)beta(1)/FN7-10 complex to a pulling force. The experiments were carried out over a three-orders-of-magnitude change in loading rate and under conditions that allowed for detection of individual alpha(5)beta(1)/FN7-10 interactions. The dynamic rupture force of the alpha(5)beta(1)/FN7-10 complex revealed two regimes of loading: a fast loading regime (>10,000 pN/s) and a slow loading regime (<10,000 pN/s) that characterize the inner and outer activation barriers of the complex, respectively. Activation by TS2/16 antibody increased both the frequency of adhesion and elevated the rupture force of the alpha(5)beta(1)/wild type FN7-10 complex to higher values in the slow loading regime. In experiments carried out with a FN7-10 RGD deleted mutant, the force measurements revealed that both inner and outer activation barriers were suppressed by the mutation. Mutations to the synergy site of FN, however, suppressed only the outer barrier activation of the complex. For both the RGD and synergy deletions, the frequency of adhesion was less than that of the wild type FN7-10, but was increased by integrin activation. The rupture force of these mutants was only slightly less than that of the wild type, and was not increased by activation. These results suggest that integrin activation involved a cooperative interaction with both the RGD and synergy sites.
Figures

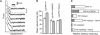

References
-
- Akiyama, S. K., and S. S. Yamada. 1985. The interaction of plasma fibronectin with fibroblastic cells in suspension. J. Biol. Chem. 260:4492–4500. - PubMed
-
- Aota, S., M. Nomizu, and K. M. Yamada. 1994. The short amino acid sequence Pro-His-Ser-Arg-Asn in human fibronectin enhances cell-adhesive function. J. Biol. Chem. 269:24756–24761. - PubMed
-
- Arroyo, A. G., A. Garcia-Pardo, and F. Sanchez-Madrid. 1993. A high affinity conformational state on VLA integrin heterodimers induced by an anti-beta 1 chain monoclonal antibody. J. Biol. Chem. 268:9863–9868. - PubMed
-
- Aukhil, I., P. Joshi, Y. Yan, and H. P. Erickson. 1993. Cell- and heparin-binding domains of the hexabrachion arm identified by tenascin expression proteins. J. Biol. Chem. 268:2542–2553. - PubMed
-
- Barillari, G., L. Albonici, S. Incerpi, L. Bogetto, G. Pistritto, A. Volpi, B. Ensoli, and V. Manzari. 2001. Inflammatory cytokines stimulate vascular smooth muscle cells locomotion and growth by enhancing alpha5beta1 integrin expression and function. Atherosclerosis. 154:377–385. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

