Single channel analysis of the regulation of GIRK1/GIRK4 channels by protein phosphorylation
- PMID: 12547819
- PMCID: PMC1302715
- DOI: 10.1016/S0006-3495(03)74954-6
Single channel analysis of the regulation of GIRK1/GIRK4 channels by protein phosphorylation
Abstract
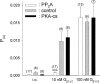
G-Protein activated, inwardly rectifying potassium channels (GIRKs) are important effectors of G-protein beta/gamma-subunits, playing essential roles in the humoral regulation of cardiac activity and also in higher brain functions. G-protein activation of channels of the GIRK1/GIRK4 heterooligomeric composition is controlled via phosphorylation by cyclic AMP dependent protein kinase (PKA) and dephosphorylation by protein phosphatase 2A (PP(2)A). To study the molecular mechanism of this unprecedented example of G-protein effector regulation, single channel recordings were performed on isolated patches of plasma membranes of Xenopus laevis oocytes. Our study shows that: (i) The open probability (P(o)) of GIRK1/GIRK4 channels, stimulated by coexpressed m(2)-receptors, was significantly increased upon addition of the catalytic subunit of PKA to the cytosolic face of an isolated membrane patch. (ii) At moderate concentrations of recombinant G(beta1/gamma2), used to activate the channel, P(o) was significantly reduced in patches treated with PP(2)A, when compared to patches with PKA-cs. (iii) Several single channel gating parameters, including modal gating behavior, were significantly different between phosphorylated and dephosphorylated channels, indicating different gating behavior between the two forms of the protein. Most of these changes were, however, not responsible for the marked difference in P(o) at moderate G-protein concentrations. (iv) An increase of the frequency of openings (f(o)) and a reduction of dwell time duration of the channel in the long-lasting C(5) state was responsible for facilitation of GIRK1/GIRK4 channels by protein phosphorylation. Dephosphorylation by PP(2)A led to an increase of G(beta1/gamma2) concentration required for full activation of the channel and hence to a reduction of the apparent affinity of GIRK1/GIRK4 for G(beta1/gamma2). (v) Although possibly not directly the target of protein phosphorylation/dephosphorylation, the last 20 C-terminal amino acids of the GIRK1 subunit are required for the reduction of apparent affinity for the G-protein by PP(2)A, indicating that they constitute an essential part of the off-switch.
Figures





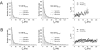

References
-
- Dascal, N., and I. Lotan. 1992. Expression of exogenous ion channels and neurotransmitter receptors in RNA-injected Xenopus oocytes. In Methods in Molecular Neurobiology, Vol. 13. A. Longstaff and P. Revest, editors. Humana Press, Totowa, New Jersey. 205–225.
-
- Dascal, N. 1997. Signalling via the G protein-activated K+ channels. Cell. Signal. 9:551–573. - PubMed
-
- Dascal, N., C. A. Doupnik, T. Ivanina, S. Bausch, W. Wang, C. Lin, J. Garvey, C. Chavkin, H. A. Lester, and N. Davidson. 1995. Inhibition of function in Xenopus oocytes of the inwardly rectifying G-protein-activated atrial K channel (GIRK1) by overexpression of a membrane-attached form of the C-terminal tail. Proc. Natl. Acad. Sci. USA. 92:6758–6762. - PMC - PubMed
-
- Dascal, N., W. Schreibmayer, N. F. Lim, W. Wang, C. Chavkin, L. DiMagno, C. Labarca, B. L. Kieffer, C. Gaveriaux-Ruff, D. Trollinger, H. A. Lester, and N. Davidson. 1993. Atrial G protein-activated K+ channel: expression cloning and molecular properties. Proc. Natl. Acad. Sci. USA. 90:10235–10239. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

