Abrogation of postentry restriction of HIV-1-based lentiviral vector transduction in simian cells
- PMID: 12547912
- PMCID: PMC298767
- DOI: 10.1073/pnas.0337541100
Abrogation of postentry restriction of HIV-1-based lentiviral vector transduction in simian cells
Abstract
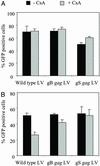
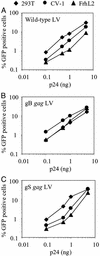
HIV-1 replication in simian cells is restricted at an early postentry step because of the presence of an inhibitory cellular factor. This block reduces the usefulness of HIV-1-based lentiviral vectors in primate animal models. Here, we demonstrate that substitution of the cyclophilin A (CyPA) binding region in the capsid of an HIV-1-based lentiviral vector (LV) with that of the macrophage tropic HIV-1 Ba-L resulted in a vector that was resistant to the inhibitory effect and efficiently transduced simian cells. Notably, the chimeric gag LV efficiently transduced primary simian hematopoietic progenitor cells, a critical cellular target in gene therapy. The alterations in the CyPA binding region did not affect CyPA incorporation; however, transduction by the gag chimeric LV seemed to be relatively insensitive to cyclosporin A, indicating that it does not require CyPA for early postentry steps. In dual infection experiments, the gag chimeric LV failed to remove the block to transduction of the WT LV, suggesting that the gag chimeric LV did not saturate the inhibitory simian cellular factor. These data suggest that the CyPA binding region of capsid contains a viral determinant involved in the postentry restriction of HIV-1-based lentiviral vectors. Overall, the findings demonstrate that the host range of HIV-1-based LV can be altered by modifications in the packaging construct.
Figures



References
-
- Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, et al. Nature. 1996;381:661–666. - PubMed
-
- Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. Nature. 1996;381:667–673. - PubMed
-
- Zack J A, Arrigo S J, Weitsman S R, Go A S, Haislip A, Chen I S. Cell. 1990;61:213–222. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

