Cell surface heparan sulfate participates in CXCL1-induced signaling
- PMID: 12549928
- PMCID: PMC2667446
- DOI: 10.1021/bi026425a
Cell surface heparan sulfate participates in CXCL1-induced signaling
Abstract
The CXC subfamily of chemokines plays an important role in diverse processes, including inflammation, wound healing, growth regulation, angiogenesis, and tumorigenesis. The ELR-CXC chemokine, CXCL1 or MGSA/GROalpha, is traditionally considered to attract neutrophils to sites of inflammation. The non-ELR-CXC chemokine, CXCL10 or IP-10, is chemotactic for monocytes, B cells, and activated T lymphocytes. In addition to its role in leukocyte migration, CXCL10 inhibits the angiogenic functions of the ELR-CXC chemokines as well as bFGF and VEGF. Heparan sulfate proteoglycans (HSPGs) are required for the interaction of bFGF and vEGF ligands and their receptors. However, the role of HSPGs in regulating the ELR-chemokines signaling and biological functions is poorly understood. We show here that the CXCL1 maximal binding to CXCR2 expressed on HEK293 and CHO-K1 cells is dependent on the presence of cell surface HSPGs. The cell surface HSPGs on cells are required for CXCL1-induced PAK1 activation. Moreover, CXCL10 can inhibit CXCL1-induced PAK1 and ERK activation as well as the CXCL1-induced chemotaxis through decreasing CXCL1 binding to cell surface heparan sulfate. These data indicate that HSPGs are involved in modulating CXCL1-induced PAK1 activation and chemotaxis through regulating CXCL1 binding activity to CXCR2 receptor. CXCL10 inhibits CXCL1-induced PAK1 activation and chemotaxis by interfering with appropriate binding of CXCL1 to CXCR2 receptor.
Figures


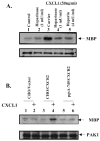

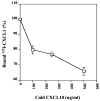
Similar articles
-
PAK1 kinase is required for CXCL1-induced chemotaxis.Biochemistry. 2002 Jun 4;41(22):7100-7. doi: 10.1021/bi025902m. Biochemistry. 2002. PMID: 12033944 Free PMC article.
-
The CXC chemokine receptor 2, CXCR2, is the putative receptor for ELR+ CXC chemokine-induced angiogenic activity.J Immunol. 2000 Nov 1;165(9):5269-77. doi: 10.4049/jimmunol.165.9.5269. J Immunol. 2000. PMID: 11046061
-
Combinatorial model of chemokine involvement in glomerular monocyte recruitment: role of CXC chemokine receptor 2 in infiltration during nephrotoxic nephritis.J Immunol. 2001 May 1;166(9):5755-62. doi: 10.4049/jimmunol.166.9.5755. J Immunol. 2001. PMID: 11313419
-
ELR+ CXC chemokines and their receptors (CXC chemokine receptor 1 and CXC chemokine receptor 2) as new therapeutic targets.Pharmacol Ther. 2006 Oct;112(1):139-49. doi: 10.1016/j.pharmthera.2006.04.002. Epub 2006 May 23. Pharmacol Ther. 2006. PMID: 16720046 Review.
-
Role of CXCL1 in tumorigenesis of melanoma.J Leukoc Biol. 2002 Jul;72(1):9-18. J Leukoc Biol. 2002. PMID: 12101257 Free PMC article. Review.
Cited by
-
The angiostatic activity of interferon-inducible protein-10/CXCL10 in human melanoma depends on binding to CXCR3 but not to glycosaminoglycan.Mol Ther. 2004 Jun;9(6):846-55. doi: 10.1016/j.ymthe.2004.01.010. Mol Ther. 2004. PMID: 15194051 Free PMC article.
-
Antibodies associated with heparin-induced thrombocytopenia (HIT) inhibit activated protein C generation: new insights into the prothrombotic nature of HIT.Blood. 2011 Sep 8;118(10):2882-8. doi: 10.1182/blood-2011-02-335208. Epub 2011 Jul 19. Blood. 2011. PMID: 21772054 Free PMC article.
-
Epigenetic Regulation of the Biosynthesis & Enzymatic Modification of Heparan Sulfate Proteoglycans: Implications for Tumorigenesis and Cancer Biomarkers.Int J Mol Sci. 2017 Jun 26;18(7):1361. doi: 10.3390/ijms18071361. Int J Mol Sci. 2017. PMID: 28672878 Free PMC article. Review.
-
Deciphering the role of glycosaminoglycans in GPCR signaling.Cell Signal. 2024 Jun;118:111149. doi: 10.1016/j.cellsig.2024.111149. Epub 2024 Mar 22. Cell Signal. 2024. PMID: 38522808 Free PMC article. Review.
-
Overcoming the inhibitory microenvironment surrounding oligodendrocyte progenitor cells following experimental demyelination.Nat Commun. 2021 Mar 26;12(1):1923. doi: 10.1038/s41467-021-22263-4. Nat Commun. 2021. PMID: 33772011 Free PMC article.
References
-
- Rossi D, Zlotnik A. Annu Rev Immunol. 2000;18:217–242. - PubMed
-
- Clark-Lewis I, Schumacher C, Baggiolini M, Moser B. J Biol Chem. 1991;266:23128–23134. - PubMed
-
- Strieter RM, Polverini PJ, Kunkel SL, Arenberg DA, Burdick MD, Kasper J, Dzuiba J, Van Damme J, Walz A, Marriott D, Chan SY, Roczniak S, Shanafelt AB. J Biol Chem. 1995;270:348–357. - PubMed
-
- Arenberg DA, Polverini PJ, Kunkel S, Shanafelt A, Hesselgesser JR, Strieter M. J Leukocyte Biol. 1997;62:554–562. - PubMed
-
- Luan J, Shattuck-Brandt R, Haghnegahdar H, Owen JD, Strieter R, Burdick M, Nirodi C, Beauchamp D, Johnson KN, Richmond A. J Leukococyte Biol. 1997;62:588–97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

