Dynactin is required for bidirectional organelle transport
- PMID: 12551954
- PMCID: PMC2172679
- DOI: 10.1083/jcb.200210066
Dynactin is required for bidirectional organelle transport
Abstract
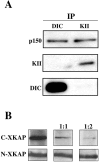
Kinesin II is a heterotrimeric plus end-directed microtubule motor responsible for the anterograde movement of organelles in various cell types. Despite substantial literature concerning the types of organelles that kinesin II transports, the question of how this motor associates with cargo organelles remains unanswered. To address this question, we have used Xenopus laevis melanophores as a model system. Through analysis of kinesin II-mediated melanosome motility, we have determined that the dynactin complex, known as an anchor for cytoplasmic dynein, also links kinesin II to organelles. Biochemical data demonstrates that the putative cargo-binding subunit of Xenopus kinesin II, Xenopus kinesin II-associated protein (XKAP), binds directly to the p150Glued subunit of dynactin. This interaction occurs through aa 530-793 of XKAP and aa 600-811 of p150Glued. These results reveal that dynactin is required for transport activity of microtubule motors of opposite polarity, cytoplasmic dynein and kinesin II, and may provide a new mechanism to coordinate their activities.
Figures

Comment in
-
Dynactin polices two-way organelle traffic.J Cell Biol. 2003 Feb 3;160(3):291-3. doi: 10.1083/jcb.200301040. J Cell Biol. 2003. PMID: 12566424 Free PMC article. Review.
References
-
- Blangy, A., L. Arnaud, and E.A. Nigg. 1997. Phosphorylation by p34cdc2 protein kinase regulates binding of the kinesin-related motor HsEg5 to the dynactin subunit p150. J. Biol. Chem. 272:19418–19424. - PubMed
-
- Cole, D.G., W.Z. Cande, R.J. Baskin, D.A. Skoufias, C.J. Hogan, and J.M. Scholey. 1992. Isolation of a sea urchin egg kinesin-related protein using peptide antibodies. J. Cell Sci. 101:291–301. - PubMed
-
- Cole, D.G., S.W. Chinn, K.P. Wedaman, K. Hall, T. Vuong, and J.M. Scholey. 1993. Novel heterotrimeric kinesin-related protein purified from sea urchin eggs. Nature. 366:268–270. - PubMed

