Characterization of a cytolytic strain of equine infectious anemia virus
- PMID: 12551976
- PMCID: PMC141072
- DOI: 10.1128/jvi.77.4.2385-2399.2003
Characterization of a cytolytic strain of equine infectious anemia virus
Abstract
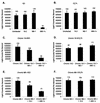
A novel strain of equine infectious anemia virus (EIAV) called vMA-1c that rapidly and specifically killed infected equine fibroblasts (ED cells) but not other infectible cell lines was established. This strain was generated from an avirulent, noncytopathic strain of EIAV, MA-1. Studies with this new cytolytic strain of virus have permitted us to define viral parameters associated with EIAV-induced cell killing and begin to explore the mechanism. vMA-1c infection resulted in induction of rapid cell death, enhanced fusogenic activity, and increased rates of spread in equine fibroblasts compared to other strains of EIAV. The highly cytolytic nature of vMA-1c suggested that this strain might be superinfecting equine fibroblasts. Receptor interference studies demonstrated that prior infection of equine fibroblasts with EIAV did not alter the ability of vMA-1c to infect and kill these cells. In similar studies in a canine fibroblast cell line, receptor interference did occur. vMA-1c infection of equine fibroblasts was also associated with large quantities of unintegrated viral DNA, a well-established hallmark of retroviral superinfection. Cloning of the vMA-1c genome identified nucleotide changes that would result in at least one amino acid change in all viral proteins. A chimeric infectious molecular clone containing the vMA-1c tat, S2, and env open reading frames recapitulated most of the characteristics of vMA-1c, including superinfection, fibroblast killing, and fusogenic activity. In summary, in vitro selection for a strain of EIAV that rapidly killed cells resulted in the generation of a virus that was able to superinfect these cells, presumably by the use of a novel mechanism of cell entry. This phenotype mapped to the 3' half of the genome.
Figures

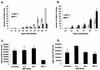



Similar articles
-
Endocytosis and a low-pH step are required for productive entry of equine infectious anemia virus.J Virol. 2005 Dec;79(23):14482-8. doi: 10.1128/JVI.79.23.14482-14488.2005. J Virol. 2005. PMID: 16282447 Free PMC article.
-
An equine infectious anemia virus variant superinfects cells through novel receptor interactions.J Virol. 2008 Oct;82(19):9425-32. doi: 10.1128/JVI.01142-08. Epub 2008 Jul 30. J Virol. 2008. PMID: 18667522 Free PMC article.
-
Equine endothelial cells support productive infection of equine infectious anemia virus.J Virol. 1998 Nov;72(11):9291-7. doi: 10.1128/JVI.72.11.9291-9297.1998. J Virol. 1998. PMID: 9765477 Free PMC article.
-
Virulence determinants of equine infectious anemia virus.Curr HIV Res. 2010 Jan;8(1):66-72. doi: 10.2174/157016210790416352. Curr HIV Res. 2010. PMID: 20210781 Review.
-
Effects on Cells.In: Baron S, editor. Medical Microbiology. 4th edition. Galveston (TX): University of Texas Medical Branch at Galveston; 1996. Chapter 44. In: Baron S, editor. Medical Microbiology. 4th edition. Galveston (TX): University of Texas Medical Branch at Galveston; 1996. Chapter 44. PMID: 21413282 Free Books & Documents. Review.
Cited by
-
Regulation of varicella-zoster virus-induced cell-to-cell fusion by the endocytosis-competent glycoproteins gH and gE.J Virol. 2004 Mar;78(6):2884-96. doi: 10.1128/jvi.78.6.2884-2896.2004. J Virol. 2004. PMID: 14990707 Free PMC article.
-
A tumor necrosis factor receptor family protein serves as a cellular receptor for the macrophage-tropic equine lentivirus.Proc Natl Acad Sci U S A. 2005 Jul 12;102(28):9918-23. doi: 10.1073/pnas.0501560102. Epub 2005 Jun 28. Proc Natl Acad Sci U S A. 2005. PMID: 15985554 Free PMC article.
-
Infection of equine monocyte-derived macrophages with an attenuated equine infectious anemia virus (EIAV) strain induces a strong resistance to the infection by a virulent EIAV strain.Vet Res. 2014 Aug 9;45(1):82. doi: 10.1186/s13567-014-0082-y. Vet Res. 2014. PMID: 25106750 Free PMC article.
-
Endocytosis and a low-pH step are required for productive entry of equine infectious anemia virus.J Virol. 2005 Dec;79(23):14482-8. doi: 10.1128/JVI.79.23.14482-14488.2005. J Virol. 2005. PMID: 16282447 Free PMC article.
-
An equine infectious anemia virus variant superinfects cells through novel receptor interactions.J Virol. 2008 Oct;82(19):9425-32. doi: 10.1128/JVI.01142-08. Epub 2008 Jul 30. J Virol. 2008. PMID: 18667522 Free PMC article.
References
-
- Anderson, M. M., A. S. Lauring, C. C. Burns, and J. Overbaugh. 2000. Identification of a cellular cofactor required for infection by feline leukemia virus. Science 287:1828-1830. - PubMed
-
- Berlioz-Torrent, C., B. L. Shacklett, L. Erdtmann, L. Delamarre, I. Bouchaert, P. Sonigo, M. C. Dokhelar, and R. Benarous. 1999. Interactions of the cytoplasmic domains of human and simian retroviral transmembrane proteins with components of the clathrin adaptor complexes modulate intracellular and cell surface expression of envelope glycoproteins. J. Virol. 73:1350-1361. - PMC - PubMed
-
- Brojatsch, J., J. Naughton, M. M. Rolls, K. Zingler, and J. A. Young. 1996. CAR1, a TNFR-related protein, is a cellular receptor for cytopathic avian leukosis-sarcoma viruses and mediates apoptosis. Cell 87:845-855. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

