TT virus in the nasal secretions of children with acute respiratory diseases: relations to viremia and disease severity
- PMID: 12551979
- PMCID: PMC141071
- DOI: 10.1128/jvi.77.4.2418-2425.2003
TT virus in the nasal secretions of children with acute respiratory diseases: relations to viremia and disease severity
Abstract
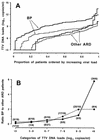
The natural history and pathogenic potential of the recently identified TT virus (TTV) are currently a matter of intensive investigation. In an attempt to shed some light on these issues, nasal and blood specimens of 1- to 24-month-old children hospitalized with a clinical diagnosis of acute respiratory disease (ARD) were examined for the presence, load, and genetic characteristics of TTV. The results have indicated that at least in young children, the respiratory tract not only represents a route by which abundant TTV can be shed into the environment but also may be a site of primary infection and continual replication. Although we found no compelling evidence that TTV was the direct cause of ARD in some of the children studied, the average loads of TTV were considerably higher in patients with bronchopneumonia (BP) than in those with milder ARD, raising interesting questions about the pathophysiological significance of TTV at this site. Furthermore, group 4 TTV was detected almost exclusively in children with BP.
Figures


References
-
- Adair, B. M. 2000. Immunopathogenesis of chicken anemia virus infection. Dev. Comp. Immunol. 24:247-255. - PubMed
-
- Ball, J. K., R. Curran, S. Berridge, A. M. Grabowska, C. L. Jameson, B. J. Thomson, W. L. Irving, and P. M. Sharp. 1999. TT virus sequence heterogeneity in vivo: evidence for co-infection with multiple genetic types. J. Gen. Virol. 80:1759-1768. - PubMed
-
- Bando, M., S. Ohno, K. Oshikawa, M. Takahashi, H. Okamoto, and Y. Sugiyama. 2001. Infection of TT virus in patients with idiopathic pulmonary fibrosis. Respir. Med. 95:935-942. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

