Lack of tumor necrosis factor alpha induces impaired proliferation of hepatitis B virus-specific cytotoxic T lymphocytes
- PMID: 12551985
- PMCID: PMC141095
- DOI: 10.1128/jvi.77.4.2469-2476.2003
Lack of tumor necrosis factor alpha induces impaired proliferation of hepatitis B virus-specific cytotoxic T lymphocytes
Abstract
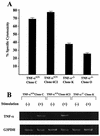
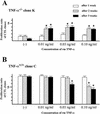
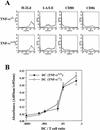
Recent studies have shown that tumor necrosis factor alpha (TNF-alpha) plays critical roles in not only viral clearance but also lymphoid tissue development and stem cell differentiation. In this study, we attempted to induce hepatitis B virus (HBV)-specific cytotoxic T lymphocytes (CTLs) by immunization of TNF-alpha knockout (TNF-alpha(-/-)) mice with HBsAg-encoding plasmid DNA. An immunization with the HBV plasmid failed to induce CTL responses in TNF-alpha(-/-) mice, although CTLs were readily induced in wild-type mice by the same protocol. Weak CTL responses were produced in TNF-alpha(-/-) mice after two sessions of immunization with the HBV plasmid; however, TNF-alpha was required to maintain the responses of these CTL lines to in vitro stimulation and, even then, the responses were lost after 3 weeks. Interestingly, a limiting dilution of a CTL line showed that HBV-specific CTL clones with high specific cytotoxicity were present in TNF-alpha(-/-) mice, but these clones again failed to proliferate for more than 3 weeks. Furthermore, since exogenously added TNF-alpha enhanced the proliferation of a TNF-alpha(-/-) clone but suppressed that of a TNF-alpha(+/+) clone in vitro, TNF-alpha also has a direct effect on the proliferation of CTLs. In conclusion, TNF-alpha is essential rather than important for the proliferation of HBV-specific CTLs both in vivo and in vitro and this effect is not only due to the activation of dendritic cells but is also induced by the direct effect on CTLs.
Figures


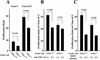
References
-
- Ando, K., K. Hiroishi, T. Kaneko, T. Moriyama, Y. Muto, N. Kayagaki, H. Yagita, K. Okumura, and M. Imawari. 1997. Perforin, Fas/Fas ligand, and TNF-α pathways as specific and bystander killing mechanisms of hepatitis C virus-specific human CTL. J. Immunol. 158:5283-5291. - PubMed
-
- Ando, K., L. G. Guidotti, S. Wirth, T. Ishikawa, G. Missale, T. Moriyama, R. D. Schreiber, H. J. Schlicht, S. N. Huang, and F. V. Chisari. 1994. Class I-restricted cytotoxic T lymphocytes are directly cytopathic for their target cells in vivo. J. Immunol. 152:3245-3253. - PubMed
-
- Banchereau, J., F. Biere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Paluckae. 2000. Immunology of dendritic cells. Annu. Rev. Immunol. 18:767-811. - PubMed
-
- Banchereau, J., and R. M. Steinman. 1998. Myeloid dendritic cells and the control of immunity. Nature 392:245-252. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

