Pseudotyped lentivirus vectors derived from simian immunodeficiency virus SIVagm with envelope glycoproteins from paramyxovirus
- PMID: 12551999
- PMCID: PMC141089
- DOI: 10.1128/jvi.77.4.2607-2614.2003
Pseudotyped lentivirus vectors derived from simian immunodeficiency virus SIVagm with envelope glycoproteins from paramyxovirus
Abstract
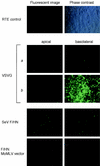
We describe the development of novel lentivirus vectors based on simian immunodeficiency virus from African green monkey (SIVagm) pseudotyped with Sendai virus (SeV) envelope glycoproteins. SeV fusion (F) and hemagglutinin-neuraminidase (HN) proteins were successfully incorporated into the SIVagm-based vector by truncation of the cytoplasmic tail of the F protein and by addition of the cytoplasmic tail of SIVagm transmembrane envelope protein to the N terminus of the HN protein. As with the vesicular stomatitis virus G glycoprotein-pseudotyped vector, the mutant SeV F- and HN-pseudotyped SIVagm vector was able to transduce various types of animal and human cell lines. Furthermore, the vector was able to transduce an enhanced green fluorescent protein reporter gene into polarized epithelial cells of rat trachea from the apical and basolateral sides. Therefore, SeV F- and HN-pseudotyped SIVagm vectors have considerable potential for effective use in gene therapy for various therapies, including respiratory diseases.
Figures


Similar articles
-
Inhibition of choroidal neovascularization via brief subretinal exposure to a newly developed lentiviral vector pseudotyped with Sendai viral envelope proteins.Hum Gene Ther. 2010 Feb;21(2):199-209. doi: 10.1089/hum.2009.102. Hum Gene Ther. 2010. PMID: 19778186
-
Lentiviral vectors pseudotyped with envelope glycoproteins derived from human parainfluenza virus type 3.Biotechnol Prog. 2004 Nov-Dec;20(6):1810-6. doi: 10.1021/bp049867h. Biotechnol Prog. 2004. PMID: 15575716
-
The Dual-Pseudotyped Lentiviral Vector with VSV-G and Sendai Virus HN Enhances Infection Efficiency through the Synergistic Effect of the Envelope Proteins.Viruses. 2024 May 23;16(6):827. doi: 10.3390/v16060827. Viruses. 2024. PMID: 38932120 Free PMC article.
-
A novel lentivirus vector derived from apathogenic simian immunodeficiency virus.Virology. 2001 Dec 20;291(2):191-7. doi: 10.1006/viro.2001.1183. Virology. 2001. PMID: 11878888
-
Naked Sendai virus vector lacking all of the envelope-related genes: reduced cytopathogenicity and immunogenicity.J Gene Med. 2006 Sep;8(9):1151-9. doi: 10.1002/jgm.938. J Gene Med. 2006. PMID: 16841365
Cited by
-
A functional henipavirus envelope glycoprotein pseudotyped lentivirus assay system.Virol J. 2010 Nov 12;7:312. doi: 10.1186/1743-422X-7-312. Virol J. 2010. PMID: 21073718 Free PMC article.
-
Intranasal Lentiviral Vector-Mediated Antibody Delivery Confers Reduction of SARS-CoV-2 Infection in Elderly and Immunocompromised Mice.Front Immunol. 2022 Apr 22;13:819058. doi: 10.3389/fimmu.2022.819058. eCollection 2022. Front Immunol. 2022. PMID: 35529866 Free PMC article.
-
Cystic Fibrosis Gene Therapy in the UK and Elsewhere.Hum Gene Ther. 2015 May;26(5):266-75. doi: 10.1089/hum.2015.027. Hum Gene Ther. 2015. PMID: 25838137 Free PMC article. Review.
-
Increased CFTR expression and function from an optimized lentiviral vector for cystic fibrosis gene therapy.Mol Ther Methods Clin Dev. 2021 Feb 27;21:94-106. doi: 10.1016/j.omtm.2021.02.020. eCollection 2021 Jun 11. Mol Ther Methods Clin Dev. 2021. PMID: 33768133 Free PMC article.
-
Envelope targeting: hemagglutinin attachment specificity rather than fusion protein cleavage-activation restricts Tupaia paramyxovirus tropism.J Virol. 2005 Aug;79(16):10155-63. doi: 10.1128/JVI.79.16.10155-10163.2005. J Virol. 2005. PMID: 16051808 Free PMC article.
References
-
- Celma, C. C. P., J. M. Manrique, J. L. Affranchino, E. Hunter, and S. A. Gonzalez. 2001. Domains in the simian immunodeficiency virus gp41 cytoplasmic tail required for envelope incorporation into particles. Virology 283:253-261. - PubMed
-
- Condit, R. C. 2001. Principles of virology, p. 19-61. In D. M. Knipe and P. M. Howley (ed.), Fields virology, Lippincott-Williams & Wilkins, Philadelphia, Pa.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

