Herpes simplex virus gE/gI expressed in epithelial cells interferes with cell-to-cell spread
- PMID: 12552008
- PMCID: PMC141120
- DOI: 10.1128/jvi.77.4.2686-2695.2003
Herpes simplex virus gE/gI expressed in epithelial cells interferes with cell-to-cell spread
Abstract
The herpes simplex virus (HSV) glycoprotein heterodimer gE/gI plays an important role in virus cell-to-cell spread in epithelial and neuronal tissues. In an analogous fashion, gE/gI promotes virus spread between certain cell types in culture, e.g., keratinocytes and epithelial cells, cells that are polarized or that form extensive cell junctions. One mechanism by which gE/gI facilitates cell-to-cell spread involves selective sorting of nascent virions to cell junctions, a process that requires the cytoplasmic domain of gE. However, the large extracellular domains of gE/gI also appear to be involved in cell-to-cell spread. Here, we show that coexpression of a truncated form of gE and gI in a human keratinocyte line, HaCaT cells, decreased the spread of HSV between cells. This truncated gE/gI was found extensively at cell junctions. Expression of wild-type gE/gI that accumulates at intracellular sites, in the trans-Golgi network, did not reduce cell-to-cell spread. There was no obvious reduction in production of infectious HSV in cells expressing gE/gI, and virus particles accumulated at cell junctions, not at intracellular sites. Expression of HSV gD, which is known to bind virus receptors, also blocked cell-to-cell spread. Therefore, like gD, gE/gI appears to be able to interact with cellular components of cell junctions, gE/gI receptors which can promote HSV cell-to-cell spread.
Figures

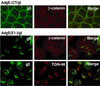
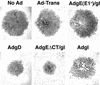
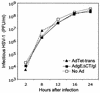
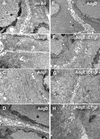
Similar articles
-
Herpes simplex virus gE/gI sorts nascent virions to epithelial cell junctions, promoting virus spread.J Virol. 2001 Jan;75(2):821-33. doi: 10.1128/JVI.75.2.821-833.2001. J Virol. 2001. PMID: 11134295 Free PMC article.
-
Cytoplasmic domain of herpes simplex virus gE causes accumulation in the trans-Golgi network, a site of virus envelopment and sorting of virions to cell junctions.J Virol. 2001 Feb;75(4):1928-40. doi: 10.1128/JVI.75.4.1928-1940.2001. J Virol. 2001. PMID: 11160692 Free PMC article.
-
Herpes simplex virus gE/gI must accumulate in the trans-Golgi network at early times and then redistribute to cell junctions to promote cell-cell spread.J Virol. 2006 Apr;80(7):3167-79. doi: 10.1128/JVI.80.7.3167-3179.2006. J Virol. 2006. PMID: 16537585 Free PMC article.
-
The role of herpes simplex virus glycoproteins in the virus replication cycle.Acta Virol. 1998 Apr;42(2):103-18. Acta Virol. 1998. PMID: 9770079 Review.
-
[Envelope and membrane glycoproteins of Herpes simplex virus].Rev Latinoam Microbiol. 1992 Jan-Mar;34(1):23-31. Rev Latinoam Microbiol. 1992. PMID: 1345300 Review. Spanish.
Cited by
-
Mucosal HSV-2 Specific CD8+ T-Cells Represent Containment of Prior Viral Shedding Rather than a Correlate of Future Protection.Front Immunol. 2013 Jul 29;4:209. doi: 10.3389/fimmu.2013.00209. eCollection 2013. Front Immunol. 2013. PMID: 23908652 Free PMC article.
-
Cell-to-cell spread inhibiting antibodies constitute a correlate of protection against herpes simplex virus type 1 reactivations: A retrospective study.Front Immunol. 2023 Mar 16;14:1143870. doi: 10.3389/fimmu.2023.1143870. eCollection 2023. Front Immunol. 2023. PMID: 37006290 Free PMC article.
-
Cellular localization of nectin-1 and glycoprotein D during herpes simplex virus infection.J Virol. 2003 Aug;77(16):8985-99. doi: 10.1128/jvi.77.16.8985-8999.2003. J Virol. 2003. PMID: 12885915 Free PMC article.
-
Cytoplasmic residues of herpes simplex virus glycoprotein gE required for secondary envelopment and binding of tegument proteins VP22 and UL11 to gE and gD.J Virol. 2007 Jan;81(1):319-31. doi: 10.1128/JVI.01842-06. Epub 2006 Oct 11. J Virol. 2007. PMID: 17035313 Free PMC article.
-
Functional interactions between herpes simplex virus pUL51, pUL7 and gE reveal cell-specific mechanisms for epithelial cell-to-cell spread.Virology. 2019 Nov;537:84-96. doi: 10.1016/j.virol.2019.08.014. Epub 2019 Aug 18. Virology. 2019. PMID: 31493658 Free PMC article.
References
-
- Balan, P., N. Davis-Poynter, S. Bell, H. Atkinson, H. Browne, and T. Minson. 1994. An analysis of the in vitro and in vivo phenotypes of mutants of herpes simplex virus type 1 lacking glycoproteins gG, gE, gI or the putative gJ. J. Gen. Virol. 75:1245-1258. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

