Structure and mechanism of a coreceptor for infection by a pathogenic feline retrovirus
- PMID: 12552012
- PMCID: PMC141074
- DOI: 10.1128/jvi.77.4.2717-2729.2003
Structure and mechanism of a coreceptor for infection by a pathogenic feline retrovirus
Abstract
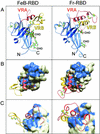
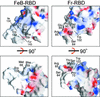
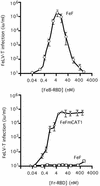
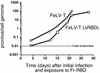
Infection of T lymphocytes by the cytopathic retrovirus feline leukemia virus subgroup T (FeLV-T) requires FeLIX, a cellular coreceptor that is encoded by an endogenous provirus and closely resembles the receptor-binding domain (RBD) of feline leukemia virus subgroup B (FeLV-B). We determined the structure of FeLV-B RBD, which has FeLIX activity, to a 2.5-A resolution by X-ray crystallography. The structure of the receptor-specific subdomain of this glycoprotein differs dramatically from that of Friend murine leukemia virus (Fr-MLV), which binds a different cell surface receptor. Remarkably, we find that Fr-MLV RBD also activates FeLV-T infection of cells expressing the Fr-MLV receptor and that FeLV-B RBD is a competitive inhibitor of infection under these conditions. These studies suggest that FeLV-T infection relies on the following property of mammalian leukemia virus RBDs: the ability to couple interaction with one of a variety of receptors to the activation of a conserved membrane fusion mechanism. A comparison of the FeLV-B and Fr-MLV RBD structures illustrates how receptor-specific regions are linked to conserved elements critical for postbinding events in virus entry.
Figures



Similar articles
-
Variable regions A and B in the envelope glycoproteins of feline leukemia virus subgroup B and amphotropic murine leukemia virus interact with discrete receptor domains.J Virol. 1997 Dec;71(12):9383-91. doi: 10.1128/JVI.71.12.9383-9391.1997. J Virol. 1997. PMID: 9371598 Free PMC article.
-
Fusion-defective gibbon ape leukemia virus vectors can be rescued by homologous but not heterologous soluble envelope proteins.J Virol. 2002 May;76(9):4267-74. doi: 10.1128/jvi.76.9.4267-4274.2002. J Virol. 2002. PMID: 11932392 Free PMC article.
-
Reduced Folate Carrier: an Entry Receptor for a Novel Feline Leukemia Virus Variant.J Virol. 2019 Jun 14;93(13):e00269-19. doi: 10.1128/JVI.00269-19. Print 2019 Jul 1. J Virol. 2019. PMID: 30996094 Free PMC article.
-
Cytopathic feline leukemia viruses cause apoptosis in hemolymphatic cells.Prog Mol Subcell Biol. 1996;16:13-43. doi: 10.1007/978-3-642-79850-4_2. Prog Mol Subcell Biol. 1996. PMID: 8822790 Review.
-
Quantification of endogenous and exogenous feline leukemia virus sequences by real-time PCR assays.Vet Immunol Immunopathol. 2008 May 15;123(1-2):129-33. doi: 10.1016/j.vetimm.2008.01.027. Epub 2008 Jan 19. Vet Immunol Immunopathol. 2008. PMID: 18295344 Review.
Cited by
-
Clinical aspects of feline retroviruses: a review.Viruses. 2012 Oct 31;4(11):2684-710. doi: 10.3390/v4112684. Viruses. 2012. PMID: 23202500 Free PMC article. Review.
-
Identification of a retroviral receptor used by an envelope protein derived by peptide library screening.Proc Natl Acad Sci U S A. 2007 Jun 26;104(26):11032-7. doi: 10.1073/pnas.0704182104. Epub 2007 Jun 20. Proc Natl Acad Sci U S A. 2007. PMID: 17581869 Free PMC article.
-
Unique Structure and Distinctive Properties of the Ancient and Ubiquitous Gamma-Type Envelope Glycoprotein.Viruses. 2023 Jan 18;15(2):274. doi: 10.3390/v15020274. Viruses. 2023. PMID: 36851488 Free PMC article. Review.
-
Comparison of the convergent receptor utilization of a retargeted feline leukemia virus envelope with a naturally-occurring porcine endogenous retrovirus A.Virology. 2012 Jun 5;427(2):118-26. doi: 10.1016/j.virol.2012.02.012. Epub 2012 Mar 8. Virology. 2012. PMID: 22405627 Free PMC article.
-
Role of Cysteines in Stabilizing the Randomized Receptor Binding Domains within Feline Leukemia Virus Envelope Proteins.J Virol. 2015 Dec 30;90(6):2971-80. doi: 10.1128/JVI.02544-15. J Virol. 2015. PMID: 26719270 Free PMC article.
References
-
- Albritton, L. M., L. Tseng, D. Scadden, and J. M. Cunningham. 1989. A putative murine ecotropic retrovirus receptor gene encodes a multiple membrane-spanning protein and confers susceptibility to virus infection. Cell 57:659-666. - PubMed
-
- Anderson, M. M., A. S. Lauring, C. C. Burns, and J. Overbaugh. 2000. Identification of a cellular cofactor required for infection by feline leukemia virus. Science 287:1828-1830. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources

