Kinetics of virus-specific CD8+ -T-cell expansion and trafficking following central nervous system infection
- PMID: 12552021
- PMCID: PMC141092
- DOI: 10.1128/jvi.77.4.2775-2778.2003
Kinetics of virus-specific CD8+ -T-cell expansion and trafficking following central nervous system infection
Abstract
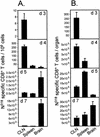
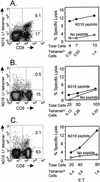
CD8+ T cells control acute infection of the central nervous system (CNS) by neurotropic mouse hepatitis virus but do not suffice to achieve sterile immunity. To determine the lag between T-cell priming and optimal activity within the CNS, the accumulation of virus-specific CD8+ T cells in the CNS relative to that in peripheral lymphoid organs was assessed by using gamma interferon-specific ELISPOT assays and class I tetramer staining. Virus-specific CD8+ T cells were first detected in the cervical lymph nodes. Expansion in the spleen was delayed and less pronounced but also preceded accumulation in the CNS. The data further suggest peripheral acquisition of cytolytic function, thus enhancing CD8+ -T-cell effector function upon cognate antigen recognition in the CNS.
Figures

References
-
- Bergmann, C. C., C. Ramakrishna, M. Kornacki, and S. A. Stohlman. 2001. Impaired T cell immunity in B cell-deficient mice following central nervous system infection. J. Immunol. 167:1575-1583. - PubMed
-
- Bergmann, C. C., J. D. Altman, D. Hinton, and S. A. Stohlman. 1999. Inverted immunodominance and impaired cytolytic function of CD8+ T cells during viral persistence in the central nervous system. J. Immunol. 163:3379-3387. - PubMed
-
- Coles, N. M., S. N. Mueller, W. R. Heath, F. R. Carbone, and A. G. Brooks. 2002. Progression of armed CTL from draining lymph node to spleen shortly after localized infection with HSV-1. J. Immunol. 168:834-838. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

