Molecular chaperone GRP78/BiP interacts with the large surface protein of hepatitis B virus in vitro and in vivo
- PMID: 12552023
- PMCID: PMC141094
- DOI: 10.1128/jvi.77.4.2784-2788.2003
Molecular chaperone GRP78/BiP interacts with the large surface protein of hepatitis B virus in vitro and in vivo
Abstract
The proper folding and assembly of viral envelope proteins are mediated by host chaperones. In this study, we demonstrated that an endoplasmic reticulum luminal chaperone GRP78/BiP bound specifically to the pre-S1 domain of the L protein in vitro and in vivo where complete viral particles were secreted, suggesting that GRP78/BiP plays an essential role in the proper folding of the L protein and/or assembly of viral envelope proteins.
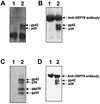
Figures



Similar articles
-
Mammalian BiP controls posttranslational ER translocation of the hepatitis B virus large envelope protein.FEBS Lett. 2008 Sep 22;582(21-22):3179-84. doi: 10.1016/j.febslet.2008.07.062. Epub 2008 Aug 15. FEBS Lett. 2008. PMID: 18708056
-
Chaperones involved in hepatitis B virus morphogenesis.Biol Chem. 1999 Mar;380(3):305-14. doi: 10.1515/BC.1999.042. Biol Chem. 1999. PMID: 10223333
-
Differential interaction of molecular chaperones with procollagen I and type IV collagen in corneal endothelial cells.Mol Vis. 2002 Jan 11;8:1-9. Mol Vis. 2002. PMID: 11815750
-
ADP-ribosylation of the molecular chaperone GRP78/BiP.Mol Cell Biochem. 1994 Sep;138(1-2):141-8. doi: 10.1007/BF00928456. Mol Cell Biochem. 1994. PMID: 7898457 Review.
-
GRP78: a chaperone with diverse roles beyond the endoplasmic reticulum.Histol Histopathol. 2008 Nov;23(11):1409-16. doi: 10.14670/HH-23.1409. Histol Histopathol. 2008. PMID: 18785123 Review.
Cited by
-
Stress proteins: the biological functions in virus infection, present and challenges for target-based antiviral drug development.Signal Transduct Target Ther. 2020 Jul 13;5(1):125. doi: 10.1038/s41392-020-00233-4. Signal Transduct Target Ther. 2020. PMID: 32661235 Free PMC article. Review.
-
Deciphering the mystery of hepatitis B virus receptors: A historical perspective.Virusdisease. 2015 Sep;26(3):97-104. doi: 10.1007/s13337-015-0260-1. Epub 2015 Jul 3. Virusdisease. 2015. PMID: 26396975 Free PMC article. Review.
-
A genetic variant of the NTCP gene is associated with HBV infection status in a Chinese population.BMC Cancer. 2016 Mar 12;16:211. doi: 10.1186/s12885-016-2257-6. BMC Cancer. 2016. PMID: 26968990 Free PMC article.
-
Expression Level of Small Envelope Protein in Addition to Sequence Divergence inside Its Major Hydrophilic Region Contributes to More Efficient Surface Antigen Secretion by Hepatitis B Virus Subgenotype D2 than Subgenotype A2.Viruses. 2020 Sep 1;12(9):967. doi: 10.3390/v12090967. Viruses. 2020. PMID: 32882910 Free PMC article.
-
GRP78 Is an Important Host Factor for Japanese Encephalitis Virus Entry and Replication in Mammalian Cells.J Virol. 2017 Feb 28;91(6):e02274-16. doi: 10.1128/JVI.02274-16. Print 2017 Mar 15. J Virol. 2017. PMID: 28053106 Free PMC article.
References
-
- Brodsky, J. L., and R. Schekman. 1994. Heat shock cognate proteins and polypeptide translocation across the endoplasmic reticulum membrane, p. 85-109. In R. I. Marimoto, A. Tissieres, and C. Georgopoulos (ed.), The biology of heat shock proteins and molecular chaperones. Cold Spring Harbor Laboratory Press, Plainview, N.Y.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

