Molecular chaperone GRP78/BiP interacts with the large surface protein of hepatitis B virus in vitro and in vivo
- PMID: 12552023
- PMCID: PMC141094
- DOI: 10.1128/jvi.77.4.2784-2788.2003
Molecular chaperone GRP78/BiP interacts with the large surface protein of hepatitis B virus in vitro and in vivo
Abstract
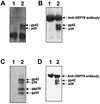
The proper folding and assembly of viral envelope proteins are mediated by host chaperones. In this study, we demonstrated that an endoplasmic reticulum luminal chaperone GRP78/BiP bound specifically to the pre-S1 domain of the L protein in vitro and in vivo where complete viral particles were secreted, suggesting that GRP78/BiP plays an essential role in the proper folding of the L protein and/or assembly of viral envelope proteins.
Figures



References
-
- Brodsky, J. L., and R. Schekman. 1994. Heat shock cognate proteins and polypeptide translocation across the endoplasmic reticulum membrane, p. 85-109. In R. I. Marimoto, A. Tissieres, and C. Georgopoulos (ed.), The biology of heat shock proteins and molecular chaperones. Cold Spring Harbor Laboratory Press, Plainview, N.Y.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

