MRI as a biomarker of disease progression in a therapeutic trial of milameline for AD
- PMID: 12552040
- PMCID: PMC2745302
- DOI: 10.1212/01.wnl.0000042480.86872.03
MRI as a biomarker of disease progression in a therapeutic trial of milameline for AD
Abstract
Objective: To assess the feasibility of using MRI measurements as a surrogate endpoint for disease progression in a therapeutic trial for AD.
Methods: A total of 362 patients with probable AD from 38 different centers participated in the MRI portion of a 52-week randomized placebo-controlled trial of milameline, a muscarinic receptor agonist. The therapeutic trial itself was not completed due to projected lack of efficacy on interim analysis; however, the MRI arm of the study was continued. Of the 362 subjects who underwent a baseline MRI study, 192 subjects underwent a second MRI 1 year later. Hippocampal volume and temporal horn volume were measured from the MRI scans.
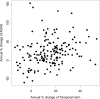
Results: The annualized percent changes in hippocampal volume (-4.9%) and temporal horn volume (16.1%) in the study patients were consistent with data from prior single-site studies. Correlations between the rate of MRI volumetric change and change in behavioral/cognitive measures were greater for the temporal horn than for the hippocampus. Decline over time was more consistently seen with imaging measures, 99% of the time for the hippocampus, than behavioral/cognitive measures (p < 0.001). Greater consistency in MRI than behavioral/clinical measures resulted in markedly lower estimated sample size requirements for clinical trials. The estimated number of subjects per arm required to detect a 50% reduction in the rate of decline over 1 year are: AD Assessment Scale-cognitive subscale 320; Mini-Mental Status Examination 241; hippocampal volume 21; temporal horn volume 54.
Conclusion: The consistency of MRI measurements obtained across sites, and the consistency between the multisite milameline data and that obtained in prior single-site studies, demonstrate the technical feasibility of using structural MRI measures as a surrogate endpoint of disease progression in therapeutic trials. However, validation of imaging as a biomarker of therapeutic efficacy in AD awaits a positive trial.
Figures
References
-
- Fox NC, Freeborough PA. Brain atrophy progression measured from registered serial MRI: validation and application to Alzheimer's disease. Journal of Magnetic Resonance Imaging. 1997;7:1069–75. - PubMed
-
- Fox NC, Scahill RI, Crum WR, Rossor MN. Correlation between rates of brain atrophy and cognitive decline in AD. Neurology. 1999;52:1687–1689. - PubMed
-
- Fox NC, Freeborough PA, Rossor MN. Visualization and quantification of rates of atrophy in Alzheimer's disease. The Lancet. 1996;348:94–97. - PubMed
-
- Fox NC, Cousens S, Scahill R, et al. Using serial registered brain magnetic resonance imaging to measure disease progression in Alzheimer disease. Arch Neurol. 2000;57:339–443. - PubMed
-
- Freeborough PA, Fox NC. The boundary shift integral: an accurate and robust measure of cerebral volume changes from registered repeat MRI. IEEE Trans on Medical Imaging. 1997;15:623–629. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
