Towards quantitative mRNA analysis in paraffin-embedded tissues using real-time reverse transcriptase-polymerase chain reaction: a methodological study on lymph nodes from melanoma patients
- PMID: 12552078
- PMCID: PMC1907376
- DOI: 10.1016/S1525-1578(10)60449-7
Towards quantitative mRNA analysis in paraffin-embedded tissues using real-time reverse transcriptase-polymerase chain reaction: a methodological study on lymph nodes from melanoma patients
Abstract
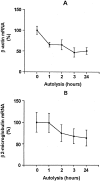
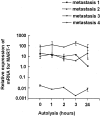
Improved extraction techniques combined with sensitive real-time reverse transcriptase-polymerase chain reaction may allow detection of mRNA in formalin-fixed, paraffin-embedded (FFPE) materials, but the factors affecting mRNA quantification in clinical material using these methods have not been systematically analyzed. We designed analyses using real-time reverse transcriptase-polymerase chain reaction for quantification of MART-1, beta-actin, and beta(2)-microglobulin mRNAs. The analytical intra- and interassay imprecision (coefficient of variation) was in the range 10 to 20% for all three genes studied. Using these protocols, we studied the influence of tissue autolysis and length of formalin-fixation on mRNA detection in metastatic melanoma. Delay in freezing reduced detectable mRNA, although this was less than predicted and mostly occurred early in autolysis. MART-1, beta-actin, and beta(2)-microglobulin mRNAs were consistently detected in FFPE metastatic melanoma even after fixation for up to 3 weeks, although the total mRNA detected was markedly reduced in fixed compared with fresh tissues (up to 99%). Quantification of MART-1 was, however, possible if this was expressed relative to a housekeeping gene. The polymerase chain reaction product from FFPE tissues could be increased up to 100-fold amplifying short (<136 bp) compared with long amplicons. Variations in time before tissue processing and in fixation length seem to be less important sources of imprecision than previously assumed. Our findings suggest that quantitative analysis of mRNA in archive and routine diagnostic tissues may be possible.
Figures



References
-
- Bustin SA: Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol 2000, 25:169-193 - PubMed
-
- Foss RD, Guha-Thakurta N, Conran RM, Gutman P: Effects of fixative and fixation time on the extraction and polymerase chain reaction amplification of RNA from paraffin-embedded tissue. Comparison of two housekeeping gene mRNA controls. Diagn Mol Pathol 1994, 3:148-155 - PubMed
-
- Krafft AE, Duncan BW, Bijwaard KE, Taubenberger JK, Lichy JH: Optimization of the isolation and amplification of RNA from formalin-fixed, paraffin-embedded tissue: the Armed Forces Institute of Pathology experience and literature review. Mol Diagn 1997, 2:217-230 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

