Estrogen inhibits GH signaling by suppressing GH-induced JAK2 phosphorylation, an effect mediated by SOCS-2
- PMID: 12552091
- PMCID: PMC298718
- DOI: 10.1073/pnas.0337600100
Estrogen inhibits GH signaling by suppressing GH-induced JAK2 phosphorylation, an effect mediated by SOCS-2
Abstract
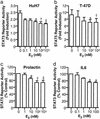
Oral estrogen administration attenuates the metabolic action of growth hormone (GH) in humans. To investigate the mechanism involved, we studied the effects of estrogen on GH signaling through Janus kinase (JAK)2 and the signal transducers and activators of transcription (STATs) in HEK293 cells stably expressing the GH receptor (293GHR), HuH7 (hepatoma) and T-47D (breast cancer) cells. 293GHR cells were transiently transfected with an estrogen receptor-alpha expression plasmid and luciferase reporters with binding elements for STAT3 and STAT5 or the beta-casein promoter. GH stimulated the reporter activities by four- to sixfold. Cotreatment with 17beta-estradiol (E(2)) resulted in a dose-dependent reduction in the response of all three reporters to GH to a maximum of 49-66% of control at 100 nM (P < 0.05). No reduction was seen when E(2) was added 1-2 h after GH treatment. Similar inhibitory effects were observed in HuH7 and T-47D cells. E(2) suppressed GH-induced JAK2 phosphorylation, an effect attenuated by actinomycin D, suggesting a requirement for gene expression. Next, we investigated the role of the suppressors of cytokine signaling (SOCS) in E(2) inhibition. E(2) increased the mRNA abundance of SOCS-2 but not SOCS-1 and SOCS-3 in HEK293 cells. The inhibitory effect of E(2) was absent in cells lacking SOCS-2 but not in those lacking SOCS-1 and SOCS-3. In conclusion, estrogen inhibits GH signaling, an action mediated by SOCS-2. This paper provides evidence for regulatory interaction between a sex steroid and the GHJAKSTAT pathway, in which SOCS-2 plays a central mechanistic role.
Figures





References
-
- Davidson M B. Endocr Rev. 1987;8:115–131. - PubMed
-
- Waters M J. In: The Handbook of Physiology. Kostyo J L, Goodman H M, editors. Vol. 5. Oxford: Oxford Univ. Press; 1999. pp. 397–444.
-
- Argetsinger L S, Campbell G S, Yang X, Witthuhn B A, Silvennoinen O, Ihle J N, Carter-Su C. Cell. 1993;74:237–244. - PubMed
-
- Finidori J. Vitam Horm (San Francisco) 2000;59:71–97. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

