Conformational dynamics of the Na+/K+-ATPase probed by voltage clamp fluorometry
- PMID: 12552111
- PMCID: PMC298709
- DOI: 10.1073/pnas.0337336100
Conformational dynamics of the Na+/K+-ATPase probed by voltage clamp fluorometry
Abstract
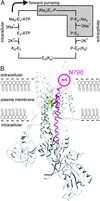
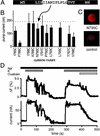
The method of voltage clamp fluorometry combined with site-directed fluorescence labeling was used to detect local protein motions of the fully active Na(+)K(+)-ATPase in real time under physiological conditions. Because helix M5 extends from the cytoplasmic site of ATP hydrolysis into the cation binding region, we chose the extracellular M5-M6 loop of the sheep alpha(1)-subunit for the insertion of cysteine residues to identify reporter positions for conformational rearrangements during the catalytic cycle. After expression of the single cysteine mutants in Xenopus oocytes and covalent attachment of tetramethylrhodamine-6-maleimide, only mutant N790C reported molecular rearrangements of the M5-M6 loop by showing large, ouabain-sensitive fluorescence changes ( approximately 5%) on addition of extracellular K(+). When the enzyme was subjected to voltage jumps under Na(+)Na(+)-exchange conditions, we observed fluorescence changes that directly correlated to transient charge movements originating from the E(1)P-E(2)P transition of the transport cycle. The voltage jump-induced fluorescence changes and transient currents were abolished after replacement of Na(+) by tetraethylammonium or on addition of ouabain, showing that conformational flexibility is impaired under these conditions. Voltage-dependent fluorescence changes could also be observed in the presence of subsaturating K(+) concentrations. This allowed to monitor the time course of voltage-dependent relaxations into a new stationary distribution of states under turnover conditions, showing the acceleration of relaxation kinetics with increasing K(+) concentrations. As a result, the stationary distribution between E(1) and E(2) states and voltage-dependent relaxation times can be determined at any time and membrane potential under Na(+)Na(+) exchange as well as Na(+)K(+) turnover conditions.
Figures

Similar articles
-
Conformational dynamics of Na+/K+- and H+/K+-ATPase probed by voltage clamp fluorometry.Ann N Y Acad Sci. 2003 Apr;986:31-8. doi: 10.1111/j.1749-6632.2003.tb07136.x. Ann N Y Acad Sci. 2003. PMID: 12763772
-
The beta subunit of the Na+/K+-ATPase follows the conformational state of the holoenzyme.J Gen Physiol. 2005 May;125(5):505-20. doi: 10.1085/jgp.200409186. J Gen Physiol. 2005. PMID: 15851504 Free PMC article.
-
Characterization of Na,K-ATPase and H,K-ATPase enzymes with glycosylation-deficient beta-subunit variants by voltage-clamp fluorometry in Xenopus oocytes.Biochemistry. 2008 Apr 8;47(14):4288-97. doi: 10.1021/bi800092k. Epub 2008 Mar 15. Biochemistry. 2008. PMID: 18341291
-
Voltage clamp fluorometry: combining fluorescence and electrophysiological methods to examine the structure-function of the Na(+)/K(+)-ATPase.Biochim Biophys Acta. 2009 Jun;1787(6):714-20. doi: 10.1016/j.bbabio.2009.03.021. Epub 2009 Apr 8. Biochim Biophys Acta. 2009. PMID: 19361481 Review.
-
Structure-function relationships of Na(+), K(+), ATP, or Mg(2+) binding and energy transduction in Na,K-ATPase.Biochim Biophys Acta. 2001 May 1;1505(1):57-74. doi: 10.1016/s0005-2728(00)00277-2. Biochim Biophys Acta. 2001. PMID: 11248189 Review.
Cited by
-
Transient Electrical Currents Mediated by the Na+/K+-ATPase: A Tour from Basic Biophysics to Human Diseases.Biophys J. 2020 Jul 21;119(2):236-242. doi: 10.1016/j.bpj.2020.06.006. Epub 2020 Jun 12. Biophys J. 2020. PMID: 32579966 Free PMC article. Review.
-
Voltage clamp fluorometric measurements on a type II Na+-coupled Pi cotransporter: shedding light on substrate binding order.J Gen Physiol. 2006 May;127(5):539-55. doi: 10.1085/jgp.200609496. J Gen Physiol. 2006. PMID: 16636203 Free PMC article.
-
Control of gastric H,K-ATPase activity by cations, voltage and intracellular pH analyzed by voltage clamp fluorometry in Xenopus oocytes.PLoS One. 2012;7(3):e33645. doi: 10.1371/journal.pone.0033645. Epub 2012 Mar 20. PLoS One. 2012. PMID: 22448261 Free PMC article.
-
Conformational changes represent the rate-limiting step in the transport cycle of maize sucrose transporter1.Plant Cell. 2013 Aug;25(8):3010-21. doi: 10.1105/tpc.113.113621. Epub 2013 Aug 20. Plant Cell. 2013. PMID: 23964025 Free PMC article.
-
Voltage Clamp Fluorometry of P-Type ATPases.Methods Mol Biol. 2016;1377:281-91. doi: 10.1007/978-1-4939-3179-8_25. Methods Mol Biol. 2016. PMID: 26695040 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

