Involvement of bone morphogenetic protein 4 (BMP-4) in pituitary prolactinoma pathogenesis through a Smad/estrogen receptor crosstalk
- PMID: 12552124
- PMCID: PMC298721
- DOI: 10.1073/pnas.0237312100
Involvement of bone morphogenetic protein 4 (BMP-4) in pituitary prolactinoma pathogenesis through a Smad/estrogen receptor crosstalk
Abstract
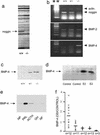
Pituitary tumor development involves clonal expansion stimulated by hormones and growth factorscytokines. Using mRNA differential display, we found that the bone morphogenetic protein (BMP) inhibitor noggin is down-regulated in prolactinomas from dopamine D2-receptor-deficient mice. BMP-4 is overexpressed in prolactinomas taken from dopamine D2-receptor-deficient female mice, but expression of the highly homologous BMP-2 does not differ in normal pituitary tissue and prolactinomas. BMP-4 is overexpressed in other prolactinoma models, including estradiol-induced rat prolactinomas and human prolactinomas, compared with normal tissue and other pituitary adenoma types (Western blot analysis of 48 tumors). BMP-4 stimulates, and noggin blocks, cell proliferation and the expression of c-Myc in human prolactinomas, whereas BMP-4 has no action in other human pituitary tumors. GH3 cells stably transfected with a dominant negative of Smad4 (Smad4dn; a BMP signal cotransducer) or noggin have reduced tumorigenicity in nude mice. Tumor growth recovered in vivo when the Smad4dn expression was lost, proving that BMP-4Smad4 are involved in tumor development in vivo. BMP-4 and estrogens act through overlapping intracellular signaling mechanisms on GH3 cell proliferation and c-myc expression: they had additive effects at low concentrations but not at saturating doses, and their action was inhibited by blocking either pathway with the reciprocal antagonist (i.e., BMP-4 with ICI 182780 or 17beta-estradiol with Smad4dn). Furthermore, coimmunoprecipitation studies demonstrate that under BMP-4 stimulation Smad4 and Smad1 physically interact with the estrogen receptor. This previously undescribed prolactinoma pathogenesis mechanism may participate in tumorigenicity in other cells where estrogens and the type beta transforming growth factor family have important roles.
Figures




References
-
- Stefaneanu L, Kovacs K, Lloyd R V, Scheithauer B W, Young W F, Jr, Sano T, Jin L. Virchows Arch B Cell Pathol Incl Mol Pathol. 1992;62:291–296. - PubMed
-
- Holmgren U, Bergstrand G, Hagenfeldt K, Werner S. Acta Endocrinol. 1986;111:452–459. - PubMed
-
- Garcia M M, Kapcala L P. J Endocrinol Invest. 1995;18:450–455. - PubMed
-
- Stefaneanu L, Kovacs K, Horvath E, Lloyd R V, Buchfelder M, Fahlbusch R, Smyth H. J Clin Endocrinol Metab. 1994;78:83–88. - PubMed
-
- Lee E J, Duan W R, Jakacka M, Gehm B D, Jameson J L. Endocrinology. 2001;142:3756–3763. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

