From Alzheimer to Huntington: why is a structural understanding so difficult?
- PMID: 12554637
- PMCID: PMC140729
- DOI: 10.1093/emboj/cdg044
From Alzheimer to Huntington: why is a structural understanding so difficult?
Abstract
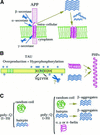
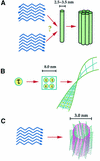
An increasing family of neurodegenerative disorders such as Alzheimer's, Parkinson's and Huntington's diseases, prion encephalopathies and cystic fibrosis is associated with aggregation of misfolded polypeptide chains which are toxic to the cell. Knowledge of the three-dimensional structure of the proteins implicated is essential for understanding why and how endogenous proteins may adopt a non-native fold. Yet, structural work has been hampered by the difficulty of handling proteins insoluble or prone to aggregation, and at the same time that is why it is interesting to study these molecules. In this review, we compare the structural knowledge accumulated for two paradigmatic misfolding disorders, Alzheimer's disease (AD) and the family of poly-glutamine diseases (poly-Q) and discuss some of the hypotheses suggested for explaining aggregate formation. While a common mechanism between these pathologies remains to be proven, a direct comparison may help in designing new strategies for approaching their study.
Figures
References
-
- Balbach J.J., Petkova,A.T., Oyler,N.A., Antzutkin,O.N., Gordon,D.J., Meredith,S.C. and Tycko,R. (2002) Supramolecular structure in full-length Alzheimer’s β-amyloid fibrils: evidence for a parallel β-sheet organization from solid-state nuclear magnetic resonance. Biophys. J., 83, 1205–1216. - PMC - PubMed
-
- Coles M., Bicknell,W., Watson,A.A., Fairlie,D.P. and Craik,D.J. (1998) Solution structure of amyloid β-peptide(1–40) in a water-micelle environment. Is the membrane-spanning domain where we think it is? Biochemistry, 37, 11064–11077. - PubMed
-
- Crescenzi O., Tomaselli,S., Guerrini,R., Salvadori,S., D’Ursi,A., Temussi,P.A. and Picone,D. (2002) Solution structure of the Alzheimer amyloid β-peptide (1–42) in an apolar microenvironment. Eur. J. Biochem., 269, 5642–5648. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

