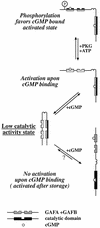
PDE5 is converted to an activated state upon cGMP binding to the GAF A domain
- PMID: 12554648
- PMCID: PMC140735
- DOI: 10.1093/emboj/cdg051
PDE5 is converted to an activated state upon cGMP binding to the GAF A domain
Abstract
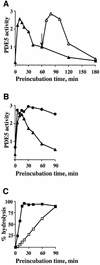
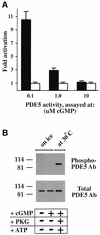
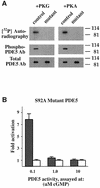
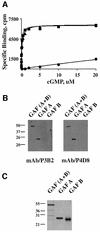
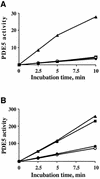
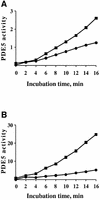
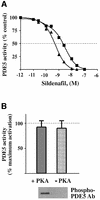
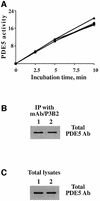
cGMP-specific, cGMP-binding phosphodiesterase (PDE5) regulates such physiological processes as smooth muscle relaxation and neuronal survival. PDE5 contains two N-terminal domains (GAF A and GAF B), but the functional roles of these domains have not been determined. Here we show that recombinant PDE5 is activated directly upon cGMP binding to the GAF A domain, and this effect does not require PDE5 phosphorylation. PDE5 exhibited time- and concentration-dependent reversible activation in response to cGMP, with the highest activation (9- to 11-fold) observed at low substrate concentrations (0.1 micro M cGMP). A monoclonal antibody directed against GAF A blocked cGMP binding, prevented PDE5 activation and decreased basal activity, revealing that PDE5 in its non-activated state has low intrinsic catalytic activity. Activated PDE5 showed higher sensitivity towards sildenafil than non-activated PDE5. The stimulatory effect of cGMP binding on the catalytic activity of PDE5 suggests that this mechanism of enzyme activation may be common among other GAF domain-containing proteins. The data also suggest that development of agonists and antagonists of PDE5 activity based on binding to this site might be possible.
Figures
Similar articles
-
A 46-amino acid segment in phosphodiesterase-5 GAF-B domain provides for high vardenafil potency over sildenafil and tadalafil and is involved in phosphodiesterase-5 dimerization.Mol Pharmacol. 2006 Nov;70(5):1822-31. doi: 10.1124/mol.106.028688. Epub 2006 Aug 22. Mol Pharmacol. 2006. PMID: 16926278
-
Hydropathic analysis and mutagenesis of the catalytic domain of the cGMP-binding cGMP-specific phosphodiesterase (PDE5). cGMP versus cAMP substrate selectivity.Biochemistry. 1998 Mar 24;37(12):4200-5. doi: 10.1021/bi972448r. Biochemistry. 1998. PMID: 9521742
-
Inhibition of cyclic GMP-binding cyclic GMP-specific phosphodiesterase (Type 5) by sildenafil and related compounds.Mol Pharmacol. 1999 Jul;56(1):124-30. doi: 10.1124/mol.56.1.124. Mol Pharmacol. 1999. PMID: 10385692
-
Mechanisms of action of PDE5 inhibition in erectile dysfunction.Int J Impot Res. 2004 Jun;16 Suppl 1:S4-7. doi: 10.1038/sj.ijir.3901205. Int J Impot Res. 2004. PMID: 15224127 Review.
-
Cyclic GMP phosphodiesterases and regulation of smooth muscle function.Circ Res. 2003 Aug 22;93(4):280-91. doi: 10.1161/01.RES.0000087541.15600.2B. Circ Res. 2003. PMID: 12933699 Review.
Cited by
-
PDE-Mediated Cyclic Nucleotide Compartmentation in Vascular Smooth Muscle Cells: From Basic to a Clinical Perspective.J Cardiovasc Dev Dis. 2021 Dec 22;9(1):4. doi: 10.3390/jcdd9010004. J Cardiovasc Dev Dis. 2021. PMID: 35050214 Free PMC article. Review.
-
Phosphodiesterase 5 inhibition in heart failure: mechanisms and clinical implications.Nat Rev Cardiol. 2009 May;6(5):349-55. doi: 10.1038/nrcardio.2009.32. Nat Rev Cardiol. 2009. PMID: 19377497 Review.
-
Compartmentation and compartment-specific regulation of PDE5 by protein kinase G allows selective cGMP-mediated regulation of platelet functions.Proc Natl Acad Sci U S A. 2008 Sep 9;105(36):13650-5. doi: 10.1073/pnas.0804738105. Epub 2008 Aug 29. Proc Natl Acad Sci U S A. 2008. PMID: 18757735 Free PMC article.
-
Intracellular cGMP increase is not involved in thyroid cancer cell death.PLoS One. 2023 Mar 30;18(3):e0283888. doi: 10.1371/journal.pone.0283888. eCollection 2023. PLoS One. 2023. PMID: 36996255 Free PMC article.
-
The role of phosphodiesterase inhibitors in the management of pulmonary vascular diseases.Glob Cardiol Sci Pract. 2014 Oct 16;2014(3):257-90. doi: 10.5339/gcsp.2014.42. eCollection 2014. Glob Cardiol Sci Pract. 2014. PMID: 25780785 Free PMC article.
References
-
- Anantharaman V., Koonin,E.V. and Aravind,L. (2001) Regulatory potential, phyletic distribution and evolution of ancient, intracellular small-molecule-binding domains. J. Mol. Biol., 307, 1271–1292. - PubMed
-
- Aravind L. and Ponting,C.P. (1997) The GAF domain: an evolutionary link between diverse phototransducing proteins. Trends Biochem. Sci., 22, 458–459. - PubMed
-
- Ballard S.A., Gingell,C.J., Tang,K., Turner,L.A., Price,M.E. and Naylor,A.M. (1998) Effects of sildenafil on the relaxation of human corpus cavernosum tissue in vitro and on the activities of cyclic nucleotide phosphodiesterase isozymes. J. Urol., 159, 2164–2171. - PubMed
-
- Corbin J.D., Turko,I.V., Beasley,A. and Francis,S.H. (2000) Phosphorylation of phosphodiesterase-5 by cyclic nucleotide-dependent protein kinase alters its catalytic and allosteric cGMP-binding activities. Eur. J. Biochem., 267, 2760–2767. - PubMed
-
- de Rooij J., Rehmann,H., van Triest,M., Cool,R.H., Wittinghofer,A. and Bos,J.L. (2000) Mechanism of regulation of the Epac family of cAMP-dependent RapGEFs. J. Biol. Chem., 275, 20829–20836. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

