Structural basis of adhesion-molecule recognition by ERM proteins revealed by the crystal structure of the radixin-ICAM-2 complex
- PMID: 12554651
- PMCID: PMC140724
- DOI: 10.1093/emboj/cdg039
Structural basis of adhesion-molecule recognition by ERM proteins revealed by the crystal structure of the radixin-ICAM-2 complex
Abstract
ERM (ezrin/radixin/moesin) proteins recognize the cytoplasmic domains of adhesion molecules in the formation of the membrane-associated cytoskeleton. Here we report the crystal structure of the radixin FERM (4.1 and ERM) domain complexed with the ICAM-2 cytoplasmic peptide. The non-polar region of the ICAM-2 peptide contains the RxxTYxVxxA sequence motif to form a beta-strand followed by a short 3(10)-helix. It binds the groove of the phosphotyrosine-binding (PTB)-like subdomain C mediated by a beta-beta association and several side-chain interactions. The binding mode of the ICAM-2 peptide to the FERM domain is distinct from that of the NPxY motif-containing peptide binding to the canonical PTB domain. Mutation analyses based on the crystal structure reveal the determinant elements of recognition and provide the first insights into the physical link between adhesion molecules and ERM proteins.
Figures
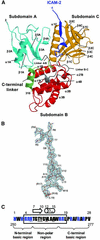
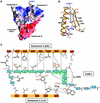


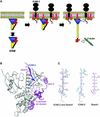

References
-
- Abrahams J.P. and Leslie,A.G.W. (1996) Methods used in the structure determination of bovine mitochondrial F1 ATPase. Acta Crystallogr. D, 52, 30–42. - PubMed
-
- Allenspach E.J. et al. (2001) ERM-dependent movement of CD43 defines a novel protein complex distal to the immunological synapse. Immunity, 15, 739–750. - PubMed
-
- Amieva M.R. and Furthmayr,H. (1995) Subcellular localization of moesin in dynamic filopodia, retraction fibers and other structures involved in substrate exploration, attachment and cell–cell contacts. Exp. Cell Res., 219, 180–196. - PubMed
-
- Andreoli C., Martin,M., Le Borgne,R., Reggio,H. and Mangeat,P. (1994) Ezrin has properties to self-associate at the plasma membrane. J. Cell Sci., 107, 2509–2521. - PubMed
-
- Arpin M., Algrain,M. and Louvard,D. (1994) Membrane–actin microfilament connections: an increasing diversity of players related to band 4.1. Curr. Opin. Cell Biol., 6, 136–141. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

